Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Wetting Properties of the CO2-Water-Calcite System via Molecular Simulations: Shape and Size Effects.
Langmuir ( IF 3.7 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.langmuir.9b02881 A Silvestri 1 , E Ataman 2 , A Budi 3 , S L S Stipp 4 , J D Gale 1 , P Raiteri 1
Langmuir ( IF 3.7 ) Pub Date : 2019-12-03 , DOI: 10.1021/acs.langmuir.9b02881 A Silvestri 1 , E Ataman 2 , A Budi 3 , S L S Stipp 4 , J D Gale 1 , P Raiteri 1
Affiliation
|
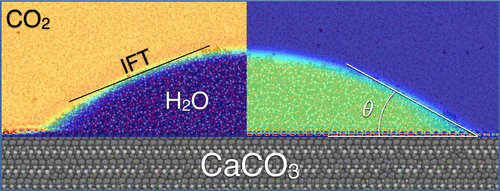
Assessment of the risks and environmental impacts of carbon geosequestration requires knowledge about the wetting behavior of mineral surfaces in the presence of CO2 and the pore fluids. In this context, the interfacial tension (IFT) between CO2 and the aqueous fluid and the contact angle, θ, with the pore mineral surfaces are the two key parameters that control the capillary pressure in the pores of the candidate host rock. Knowledge of these two parameters and their dependence on the local conditions of pressure, temperature, and salinity is essential for the correct prediction of structural and residual trapping. We have performed classical molecular dynamics simulations to predict the CO2-water IFT and the CO2-water-calcite contact angle. The IFT results are consistent with previous simulations, where simple point charge water models have been shown to underestimate the water surface tension, thus affecting the simulated IFT values. When combined with the EPM2 CO2 model, the SPC/Fw water model indeed underestimates the IFT in the low-pressure region at all temperatures studied. On the other hand, at high pressure and low temperature, the IFT is overestimated by ∼5 mN/m. Literature data regarding the CO2/water/calcite contact angle on calcite are contradictory. Using our new set of force field parameters, we performed NVT simulations at 323 K and 20 MPa to calculate the contact angle of a water droplet on the calcite {10.4} surface in a CO2 atmosphere. We performed simulations for both spherical and cylindrical droplet configurations for different initial radii to study the size dependence of the water contact angle on calcite in the presence of CO2. Our results suggest that the contact angle of a cylindrical droplet, is independent of droplet size, for droplets with a radius of 50 Å or more. On the contrary, spherical droplets make a contact angle that is strongly influenced by their size. At the largest size explored in this study, both spherical and cylindrical droplets converge to the same contact angle, 38°, indicating that calcite is strongly wetted by water.
中文翻译:

通过分子模拟对CO2-水-方解石体系的润湿特性:形状和尺寸效应。
碳固存的风险和环境影响的评估需要了解在存在CO2和孔隙流体的情况下矿物表面的润湿行为。在这种情况下,CO2与含水流体之间的界面张力(IFT)以及与孔隙矿物表面的接触角θ是控制候选基质岩石孔隙中毛细压力的两个关键参数。了解这两个参数及其对压力,温度和盐度局部条件的依赖性,对于正确预测结构和残留捕集至关重要。我们已经进行了经典的分子动力学模拟,以预测CO2-水的IFT和CO2-水-方解石的接触角。IFT结果与以前的模拟一致,其中简单的点进水模型已经显示出低估了水的表面张力,从而影响了模拟的IFT值。当与EPM2 CO2模型结合使用时,在所有研究温度下,SPC / Fw水模型确实低估了低压区的IFT。另一方面,在高压和低温下,IFT被高估了约5 mN / m。关于CO2 /水/方解石与方解石接触角的文献数据是矛盾的。使用我们新的力场参数集,我们在323 K和20 MPa下执行了NVT模拟,以计算水滴在CO2气氛中方解石{10.4}表面上的接触角。我们对不同初始半径的球形和圆柱形液滴构型进行了仿真,以研究在CO2存在下水接触角对方解石的尺寸依赖性。我们的结果表明,对于半径为50或更大的液滴,圆柱形液滴的接触角与液滴大小无关。相反,球形液滴的接触角受其大小的影响很大。在这项研究中探索的最大尺寸下,球形和圆柱形液滴均会聚到相同的接触角38°,这表明方解石被水强烈润湿。球形液滴的接触角受其大小的影响很大。在这项研究中探索的最大尺寸下,球形和圆柱形液滴均会聚到相同的接触角38°,这表明方解石被水强烈润湿。球形液滴的接触角受其大小的影响很大。在这项研究中探索的最大尺寸下,球形和圆柱形液滴均会聚到相同的接触角38°,这表明方解石被水强烈润湿。
更新日期:2019-12-04
中文翻译:

通过分子模拟对CO2-水-方解石体系的润湿特性:形状和尺寸效应。
碳固存的风险和环境影响的评估需要了解在存在CO2和孔隙流体的情况下矿物表面的润湿行为。在这种情况下,CO2与含水流体之间的界面张力(IFT)以及与孔隙矿物表面的接触角θ是控制候选基质岩石孔隙中毛细压力的两个关键参数。了解这两个参数及其对压力,温度和盐度局部条件的依赖性,对于正确预测结构和残留捕集至关重要。我们已经进行了经典的分子动力学模拟,以预测CO2-水的IFT和CO2-水-方解石的接触角。IFT结果与以前的模拟一致,其中简单的点进水模型已经显示出低估了水的表面张力,从而影响了模拟的IFT值。当与EPM2 CO2模型结合使用时,在所有研究温度下,SPC / Fw水模型确实低估了低压区的IFT。另一方面,在高压和低温下,IFT被高估了约5 mN / m。关于CO2 /水/方解石与方解石接触角的文献数据是矛盾的。使用我们新的力场参数集,我们在323 K和20 MPa下执行了NVT模拟,以计算水滴在CO2气氛中方解石{10.4}表面上的接触角。我们对不同初始半径的球形和圆柱形液滴构型进行了仿真,以研究在CO2存在下水接触角对方解石的尺寸依赖性。我们的结果表明,对于半径为50或更大的液滴,圆柱形液滴的接触角与液滴大小无关。相反,球形液滴的接触角受其大小的影响很大。在这项研究中探索的最大尺寸下,球形和圆柱形液滴均会聚到相同的接触角38°,这表明方解石被水强烈润湿。球形液滴的接触角受其大小的影响很大。在这项研究中探索的最大尺寸下,球形和圆柱形液滴均会聚到相同的接触角38°,这表明方解石被水强烈润湿。球形液滴的接触角受其大小的影响很大。在这项研究中探索的最大尺寸下,球形和圆柱形液滴均会聚到相同的接触角38°,这表明方解石被水强烈润湿。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号