当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A New Way of Belonging: Active-Site Investigation of L-DOPA Dioxygenase, a VOC Family Enzyme from Lincomycin Biosynthesis.
Biochemistry ( IF 2.9 ) Pub Date : 2019-11-18 , DOI: 10.1021/acs.biochem.9b00456 Keri L Colabroy 1 , Alyssa D Horwitz 1 , Victoria R Basciano 1 , Yizhi Fu 1 , Kelly M Travitz 1 , Miranda K Robinson 1 , Brittany A Shimanski 1 , Thomas W Hoffmann 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-11-18 , DOI: 10.1021/acs.biochem.9b00456 Keri L Colabroy 1 , Alyssa D Horwitz 1 , Victoria R Basciano 1 , Yizhi Fu 1 , Kelly M Travitz 1 , Miranda K Robinson 1 , Brittany A Shimanski 1 , Thomas W Hoffmann 1
Affiliation
|
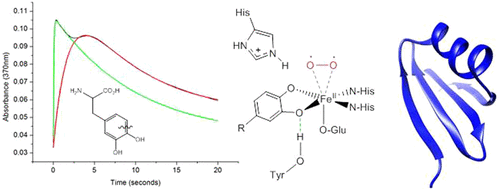
Extradiol dioxygenase chemistry is essential for catechol breakdown. The largest natural reservoir of catechols, or 1,2-dihydroxybenzenes, is the plant woody-tissue polymer lignin. Vicinal–oxygen–chelate (VOC) dioxygenases make up the largest group of characterized extradiol dioxygenases, and while most are found as part of catabolic pathways degrading a variety of natural and human-made aromatic rings, L-DOPA (l-3,4-dihydroxyphenylalanine) dioxygenase is a VOC enzyme that participates in the biosynthesis of a natural product. All VOC superfamily members shared conserved elements of catalysis, yet despite decades of investigation of VOC enzymes, the relationships between VOC domain architecture and enzymatic function remain complex and poorly understood. Herein, we present evidence that L-DOPA dioxygenase is the representative member of a new topological class of VOC extradiol dioxygenases. Guided by its evolutionary similarity to glyoxylase enzymes, we performed a careful investigation of the Streptomyces lincolnensis L-DOPA dioxygenase (LmbB1) active site through mutagenesis, kinetic, and pH studies. Our results demonstrate that the L-DOPA dioxygenase reaction depends upon an active-site tyrosine and histidine and is remarkably resilient to mutation, even at the iron-ligating residues. Evaluation of the cleavage reaction as a function of pH supports the role of a histidine in acid–base catalysis. The active-site architecture is functionally consistent with the existing knowledge of VOC extradiol dioxygenase catalysis.
中文翻译:

一种新的归属方式:L-DOPA双加氧酶(一种来自林可霉素生物合成的VOC家族酶)的主动站点研究。
外二醇双加氧酶的化学作用对于分解儿茶酚至关重要。儿茶酚或1,2-二羟基苯的最大天然贮藏库是植物木质组织聚合物木质素。邻二氧合螯合(VOC)双加氧酶构成了特征化的胞外二醇双加氧酶的最大类别,虽然大多数被发现是分解代谢途径的一部分,但降解了各种天然和人为的芳香环,L-DOPA(l-3,4-二羟基苯丙氨酸)双加氧酶是一种VOC酶,参与天然产物的生物合成。所有VOC超家族成员都共享保守的催化元素,尽管对VOC酶进行了数十年的研究,但VOC结构域与酶功能之间的关系仍然很复杂,人们对此知之甚少。在本文中,我们提供证据表明L-DOPA双加氧酶是VOC外二醇双加氧酶的新拓扑类别的代表成员。以其与乙醛酸酶的进化相似性为指导,我们对林肯链霉菌进行了仔细的研究。L-DOPA双加氧酶(LmbB1)的活性位点通过诱变,动力学和pH研究。我们的结果表明,L-DOPA双加氧酶反应取决于活性位点酪氨酸和组氨酸,即使在铁连接残基上也能显着地抵抗突变。裂解反应与pH的函数关系的评估支持了组氨酸在酸碱催化中的作用。活性部位的结构在功能上与VOC外二醇双加氧酶催化的现有知识相一致。
更新日期:2019-11-18
中文翻译:

一种新的归属方式:L-DOPA双加氧酶(一种来自林可霉素生物合成的VOC家族酶)的主动站点研究。
外二醇双加氧酶的化学作用对于分解儿茶酚至关重要。儿茶酚或1,2-二羟基苯的最大天然贮藏库是植物木质组织聚合物木质素。邻二氧合螯合(VOC)双加氧酶构成了特征化的胞外二醇双加氧酶的最大类别,虽然大多数被发现是分解代谢途径的一部分,但降解了各种天然和人为的芳香环,L-DOPA(l-3,4-二羟基苯丙氨酸)双加氧酶是一种VOC酶,参与天然产物的生物合成。所有VOC超家族成员都共享保守的催化元素,尽管对VOC酶进行了数十年的研究,但VOC结构域与酶功能之间的关系仍然很复杂,人们对此知之甚少。在本文中,我们提供证据表明L-DOPA双加氧酶是VOC外二醇双加氧酶的新拓扑类别的代表成员。以其与乙醛酸酶的进化相似性为指导,我们对林肯链霉菌进行了仔细的研究。L-DOPA双加氧酶(LmbB1)的活性位点通过诱变,动力学和pH研究。我们的结果表明,L-DOPA双加氧酶反应取决于活性位点酪氨酸和组氨酸,即使在铁连接残基上也能显着地抵抗突变。裂解反应与pH的函数关系的评估支持了组氨酸在酸碱催化中的作用。活性部位的结构在功能上与VOC外二醇双加氧酶催化的现有知识相一致。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号