当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Determining the Critical Micelle Concentration of Surfactants by a Simple and Fast Titration Method.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-02-26 , DOI: 10.1021/acs.analchem.9b04638 Shengen Wu 1 , Feiqing Liang 1 , Danna Hu 1 , Hao Li 1 , Weijie Yang 1 , Qiuhua Zhu 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-02-26 , DOI: 10.1021/acs.analchem.9b04638 Shengen Wu 1 , Feiqing Liang 1 , Danna Hu 1 , Hao Li 1 , Weijie Yang 1 , Qiuhua Zhu 1
Affiliation
|
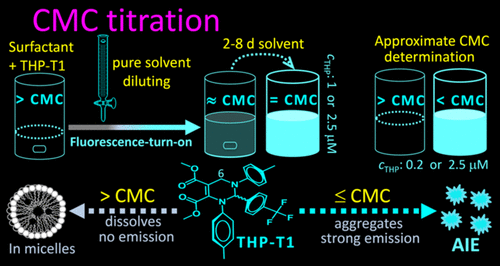
Critical micelle concentration (CMC) is a crucial parameter of widely used surfactants, and many methods have been developed for CMC determination. However, the current methods for CMC determination, such as conductive, surface tension, and fluorometric methods, are tedious and time- and sample-consuming because a series of samples with different concentrations of surfactants need to be prepared and measured. Although an economical, simple, and fast titration method for CMC determination (only one sample and several minutes are needed) was reported using changes in the color/fluorescence of ionic organic dyes, it has not been used in practical CMC determination owing to the disadvantages of these dyes: very narrow application range (only suitable for cationic or anionic surfactants) and difficult to identify titration end point, especially using different concentrations (10-300 μM) for the same kind surfactants. Here a C6-unsubstituted tetrahydropyrimidine (THP-T1) was found to possess unique and excellent characteristics in titrated surfactant solutions: above CMC, preferring to dissolve in micelles and showing no emission, and not until near/at CMC, being released from micelles and instantly forming aggregates with strong fluorescence. The fluorescence-turn-on change at CMC (titration end point) is so sensitive that it can be clearly observed without comparison of blank and control of dye concentration, and the concentration (c'THP) of THP-T1 in titrated solution at CMC is only about 1 μM for zwitterionic surfactants and 2.5 μM for other kinds of surfactants. The CMC values determined by the THP-T1-based titration method are almost the same as those determined by the fluorometric method using THP-T1 as probe. THP-T1 overcomes the disadvantages of reported dyes for CMC titration and realizes the economical, simple and fast CMC titration of different kinds of surfactants for the first time.
中文翻译:

通过简单和快速的滴定方法确定表面活性剂的临界胶束浓度。
临界胶束浓度(CMC)是广泛使用的表面活性剂的关键参数,并且已经开发出许多用于CMC测定的方法。但是,由于需要准备和测量一系列具有不同浓度的表面活性剂的样品,因此用于CMC测定的当前方法(例如导电,表面张力和荧光法)既繁琐又耗时且耗费样品。尽管据报道使用离子有机染料的颜色/荧光变化来测定CMC的经济,简单,快速的滴定方法(仅需一个样品和几分钟),但由于其缺点,该方法尚未用于实际的CMC测定中。这些染料:应用范围非常狭窄(仅适用于阳离子或阴离子表面活性剂),并且难以确定滴定终点,特别是对于相同类型的表面活性剂,使用不同的浓度(10-300μM)。在此发现C6-未取代的四氢嘧啶(THP-T1)在滴定的表面活性剂溶液中具有独特而优异的特性:在CMC上方,倾向于溶解在胶束中并且没有发射,直到CMC附近/附近才从胶束中释放出来。立即形成具有强荧光的聚集体。CMC(滴定终点)处的荧光开启变化非常敏感,因此无需比较空白和染料浓度以及CMC滴定溶液中THP-T1的浓度(c'THP),就可以清楚地观察到它。两性离子表面活性剂仅为约1μM,而其他种类的表面活性剂仅为2.5μM。通过基于THP-T1的滴定法确定的CMC值与通过使用THP-T1作为探针的荧光法确定的CMC值几乎相同。THP-T1克服了已报道的染料用于CMC滴定的缺点,并首次实现了经济,简便,快速的各种表面活性剂CMC滴定。
更新日期:2020-02-26
中文翻译:

通过简单和快速的滴定方法确定表面活性剂的临界胶束浓度。
临界胶束浓度(CMC)是广泛使用的表面活性剂的关键参数,并且已经开发出许多用于CMC测定的方法。但是,由于需要准备和测量一系列具有不同浓度的表面活性剂的样品,因此用于CMC测定的当前方法(例如导电,表面张力和荧光法)既繁琐又耗时且耗费样品。尽管据报道使用离子有机染料的颜色/荧光变化来测定CMC的经济,简单,快速的滴定方法(仅需一个样品和几分钟),但由于其缺点,该方法尚未用于实际的CMC测定中。这些染料:应用范围非常狭窄(仅适用于阳离子或阴离子表面活性剂),并且难以确定滴定终点,特别是对于相同类型的表面活性剂,使用不同的浓度(10-300μM)。在此发现C6-未取代的四氢嘧啶(THP-T1)在滴定的表面活性剂溶液中具有独特而优异的特性:在CMC上方,倾向于溶解在胶束中并且没有发射,直到CMC附近/附近才从胶束中释放出来。立即形成具有强荧光的聚集体。CMC(滴定终点)处的荧光开启变化非常敏感,因此无需比较空白和染料浓度以及CMC滴定溶液中THP-T1的浓度(c'THP),就可以清楚地观察到它。两性离子表面活性剂仅为约1μM,而其他种类的表面活性剂仅为2.5μM。通过基于THP-T1的滴定法确定的CMC值与通过使用THP-T1作为探针的荧光法确定的CMC值几乎相同。THP-T1克服了已报道的染料用于CMC滴定的缺点,并首次实现了经济,简便,快速的各种表面活性剂CMC滴定。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号