当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Using Dialkylimidazolium Ionic Liquids To Break the Methanol + Methyl Acetate Azeotrope
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-11-22 , DOI: 10.1021/acs.iecr.9b05760 Joshua M. Winnert 1 , V. K. P. Janakey Devi 1 , Joan F. Brennecke 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-11-22 , DOI: 10.1021/acs.iecr.9b05760 Joshua M. Winnert 1 , V. K. P. Janakey Devi 1 , Joan F. Brennecke 1
Affiliation
|
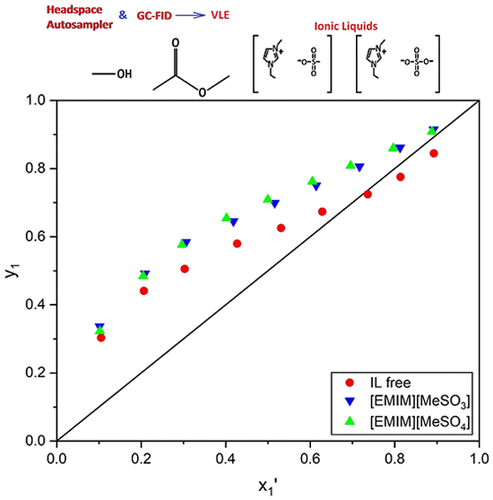
Separating mixtures that form azeotropes or have relative volatilities close to 1.0 has always been a challenging problem. Conventional distillation cannot yield high purity products for these systems, so separating them often becomes a capital- and energy-intensive process. One of the most popular alternative methods used to accomplish these separations is extractive distillation, in which an entrainer is added to increase the relative volatility of the mixture. Ionic liquids (ILs) show great promise as entrainers due to their extremely low volatility, thermal stability, and chemical diversity. In this work, 1-ethyl-3-methylimidazolium methanesulfonate ([EMIM][MeSO3]) and 1-ethyl-3-methylimidazolium methylsulfate ([EMIM][MeSO4]) were investigated for their ability to break the methyl acetate + methanol azeotrope at 313.15 K. [EMIM][MeSO3] was able to break the azeotrope at just 2.5 mol % IL, the lowest value currently recorded in the literature. [EMIM][MeSO4] also performed well, breaking the azeotrope at 6.0 mol %. Finally, the Non-Random Two-Liquid (NRTL) model was used to correlate the vapor–liquid equilibrium (VLE) data of the binary systems and then predict the ternary phase behavior of systems with ionic liquids.
中文翻译:

使用二烷基咪唑鎓离子液体破坏甲醇+乙酸甲酯共沸物
分离形成共沸物或相对挥发度接近1.0的混合物一直是一个具有挑战性的问题。常规蒸馏不能为这些系统产生高纯度的产物,因此分离它们通常成为资本和能量密集的过程。用于完成这些分离的最流行的替代方法之一是萃取蒸馏,其中加入夹带剂以增加混合物的相对挥发性。离子液体(ILs)具有极低的挥发性,热稳定性和化学多样性,因此有望作为夹带剂。在这项工作中,甲基磺酸1-乙基-3-甲基咪唑鎓([EMIM] [MeSO 3 ])和甲基丙烯酸1-乙基-3-甲基咪唑鎓([EMIM] [MeSO 4 ]])研究了它们在313.15 K时破坏乙酸甲酯+甲醇共沸物的能力。[EMIM] [MeSO 3 ]仅在2.5 mol%IL时就能够破坏该共沸物,这是目前文献中记录的最低值。[EMIM] [MeSO 4 ]也表现良好,以6.0摩尔%的比例破坏了恒沸物。最后,使用非随机两液(NRTL)模型来关联二元系统的气液平衡(VLE)数据,然后预测具有离子液体的系统的三元相行为。
更新日期:2019-11-28
中文翻译:

使用二烷基咪唑鎓离子液体破坏甲醇+乙酸甲酯共沸物
分离形成共沸物或相对挥发度接近1.0的混合物一直是一个具有挑战性的问题。常规蒸馏不能为这些系统产生高纯度的产物,因此分离它们通常成为资本和能量密集的过程。用于完成这些分离的最流行的替代方法之一是萃取蒸馏,其中加入夹带剂以增加混合物的相对挥发性。离子液体(ILs)具有极低的挥发性,热稳定性和化学多样性,因此有望作为夹带剂。在这项工作中,甲基磺酸1-乙基-3-甲基咪唑鎓([EMIM] [MeSO 3 ])和甲基丙烯酸1-乙基-3-甲基咪唑鎓([EMIM] [MeSO 4 ]])研究了它们在313.15 K时破坏乙酸甲酯+甲醇共沸物的能力。[EMIM] [MeSO 3 ]仅在2.5 mol%IL时就能够破坏该共沸物,这是目前文献中记录的最低值。[EMIM] [MeSO 4 ]也表现良好,以6.0摩尔%的比例破坏了恒沸物。最后,使用非随机两液(NRTL)模型来关联二元系统的气液平衡(VLE)数据,然后预测具有离子液体的系统的三元相行为。

































 京公网安备 11010802027423号
京公网安备 11010802027423号