当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Barium Vanadate BaxV2O5 (x ≈ 0.16) for Fast Lithium Intercalation: Lower Symmetry for Higher Flexibility and Electrochemical Durability
Small Methods ( IF 10.7 ) Pub Date : 2019-11-11 , DOI: 10.1002/smtd.201900585 Jiliang Zhang,Hangkong Li,Chang‐Zhong Liao,Vincent Wing‐hei Lau,Kam Wa Wong,Chung‐Kai Chang,Hwo‐Shuenn Sheu,Kaimin Shih,Yong‐Mook Kang
Small Methods ( IF 10.7 ) Pub Date : 2019-11-11 , DOI: 10.1002/smtd.201900585 Jiliang Zhang,Hangkong Li,Chang‐Zhong Liao,Vincent Wing‐hei Lau,Kam Wa Wong,Chung‐Kai Chang,Hwo‐Shuenn Sheu,Kaimin Shih,Yong‐Mook Kang
|
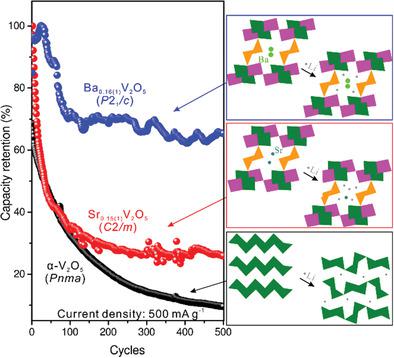
Na0.33V2O5‐type metal vanadates generally show better cycling stability than α‐V2O5 as a cathode in Li‐ion batteries, because they contain enough crystallographic voids that allow for lithium intercalation without significant structural deformation, but metal leaching upon cycling deteriorates their capacity retention. Here a new barium vanadate Ba0.16(1)V2O5 is reported that does not undergo Ba leaching during cycling and shows a great promise for fast Li intercalation. Sr0.15(1)V2O5 is isostructural to Na0.33V2O5 (space group C2/m), while Ba0.16(1)V2O5 adopts a new structure (space group P21/c), a derivative of Na0.33V2O5. The framework of Ba0.16(1)V2O5 consists of edge‐shared VO6 octahedral layers and the VO5 pyramidal bridge, generating unidirectional tunnels. Unlike the in‐plane arrangement of atoms in Sr0.15(1)V2O5, the nonplanar arrangement in Ba0.16(1)V2O5 makes the host framework more flexible and creates larger voids for Ba. Compared with the combination of displacement and intercalation mechanisms in the Sr0.15(1)V2O5 cathode, Li intercalation looks dominant in the Ba0.16(1)V2O5 cathode due to its increased flexibility. Hence, Ba0.16(1)V2O5 exhibits improved cycling stability compared to Sr0.15(1)V2O5, suggesting that lower symmetry leads to higher cyclic stability in the V2O5‐related compounds. This study illustrates how guest cations can tune the structural symmetry to modulate the reaction mechanism of electrode materials toward superior performances.
中文翻译:

用于快速锂嵌入的新型钒酸钡BaxV2O5(x≈0.16):较低的对称性,具有更高的柔韧性和电化学耐久性
的Na 0.33 V 2 ø 5型金属通常钒酸盐表现出更好的循环稳定性比α-V 2 ø 5如在锂离子电池的阴极,因为它们含有足够的晶体空隙,其允许锂嵌入而不显著结构变形,但金属浸出循环时会降低其容量保持能力。此处报道了一种新的钒酸钡Ba 0.16(1) V 2 O 5,其在循环过程中未经历Ba浸出,并显示出实现快速Li嵌入的巨大希望。Sr 0.15(1) V 2 O 5与Na 0.33 V 2同构O 5(空间组C 2 / m),而Ba 0.16(1) V 2 O 5采用新结构(空间组P 2 1 / c),即Na 0.33 V 2 O 5的衍生物。Ba 0.16(1) V 2 O 5的框架由边缘共享的VO 6八面体层和VO 5金字塔形桥组成,生成单向隧道。与Sr 0.15(1) V 2 O 5中原子的平面排列不同,Ba 0.16(1) V 2 O 5中的非平面排列使主体框架更加灵活,并为Ba创建了较大的空隙。与在Sr 0.15(1) V 2 O 5阴极中置换和嵌入机制的组合相比,Li嵌入在Ba 0.16(1) V 2 O 5阴极中占主导地位,因为它具有更高的柔韧性。因此,与Sr 0.15(1) V 2 O 5相比,Ba 0.16(1) V 2 O 5表现出改善的循环稳定性。,表明较低的对称性会导致V 2 O 5相关化合物的循环稳定性更高。这项研究说明了客体阳离子如何调节结构对称性,以调节电极材料的反应机理,从而达到更高的性能。
更新日期:2019-11-11
中文翻译:

用于快速锂嵌入的新型钒酸钡BaxV2O5(x≈0.16):较低的对称性,具有更高的柔韧性和电化学耐久性
的Na 0.33 V 2 ø 5型金属通常钒酸盐表现出更好的循环稳定性比α-V 2 ø 5如在锂离子电池的阴极,因为它们含有足够的晶体空隙,其允许锂嵌入而不显著结构变形,但金属浸出循环时会降低其容量保持能力。此处报道了一种新的钒酸钡Ba 0.16(1) V 2 O 5,其在循环过程中未经历Ba浸出,并显示出实现快速Li嵌入的巨大希望。Sr 0.15(1) V 2 O 5与Na 0.33 V 2同构O 5(空间组C 2 / m),而Ba 0.16(1) V 2 O 5采用新结构(空间组P 2 1 / c),即Na 0.33 V 2 O 5的衍生物。Ba 0.16(1) V 2 O 5的框架由边缘共享的VO 6八面体层和VO 5金字塔形桥组成,生成单向隧道。与Sr 0.15(1) V 2 O 5中原子的平面排列不同,Ba 0.16(1) V 2 O 5中的非平面排列使主体框架更加灵活,并为Ba创建了较大的空隙。与在Sr 0.15(1) V 2 O 5阴极中置换和嵌入机制的组合相比,Li嵌入在Ba 0.16(1) V 2 O 5阴极中占主导地位,因为它具有更高的柔韧性。因此,与Sr 0.15(1) V 2 O 5相比,Ba 0.16(1) V 2 O 5表现出改善的循环稳定性。,表明较低的对称性会导致V 2 O 5相关化合物的循环稳定性更高。这项研究说明了客体阳离子如何调节结构对称性,以调节电极材料的反应机理,从而达到更高的性能。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号