当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Diagram of AlCl3–FeCl3–H2O(−HCl) Salt Water System at 298.15 K and Its Application in the Crystallization of AlCl3·6H2O
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-11-11 , DOI: 10.1021/acs.jced.9b00238 Huaigang Cheng 1 , Lixiang Wu 1 , Liqiong Cao 1 , Jing Zhao 1, 2 , Fangbin Xue 1 , Fangqin Cheng 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-11-11 , DOI: 10.1021/acs.jced.9b00238 Huaigang Cheng 1 , Lixiang Wu 1 , Liqiong Cao 1 , Jing Zhao 1, 2 , Fangbin Xue 1 , Fangqin Cheng 1
Affiliation
|
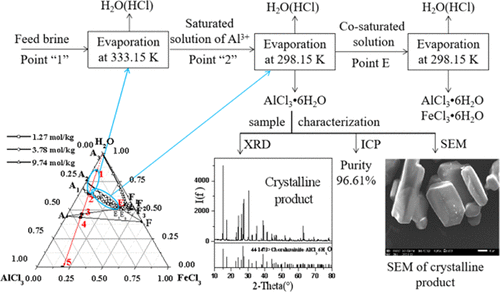
Aluminum–ferric separation has attracted significant interest in the field of hydrometallurgy. Using phase equilibria theory, fractional crystallization and separation of a mixed salt solution is a relatively effective method. The phase equilibria of the ternary system or tertiary system AlCl3–FeCl3–H2O(−HCl) at 298.15 K for different H+ concentrations were studied using the isothermal dissolution equilibrium method, and plotted the corresponding phase diagrams and density diagrams. The experimental results show that with increasing H+ concentration, the solubility of AlCl3 decreased from 31.20 to 0.13%, and the crystallization region of AlCl3·6H2O gradually increased. H+ concentration had little influence on FeCl3 solubility, and the solubility of FeCl3 was 47.52%. Through theoretical calculations, the desired amount of water to evaporate and crystal precipitation can be obtained through the calculation model for the system composed of different materials. The route is designed by the phase equilibrium and the phase diagram theory, and the separation of aluminum and ferric from aluminate acid leaching solution of coal gangue in literature. The direct yield of AlCl3·6H2O obtained by evaporation and crystallization was 77.15%, and the purity reached 99.81% after rinsing with concentrated hydrochloric acid.
中文翻译:

AlCl 3 -FeCl 3 -H 2 O(-HCl)盐水系统在298.15 K时的相图及其在AlCl 3 ·6H 2 O结晶中的应用
铝铁分离在湿法冶金领域引起了极大的兴趣。使用相平衡理论,分步结晶和分离混合盐溶液是一种相对有效的方法。采用等温溶出平衡法研究了不同H +浓度下三元体系或三元体系AlCl 3 -FeCl 3 -H 2 O(-HCl)在298.15 K时的相平衡,并绘制了相应的相图和密度图。实验结果表明,随着H +浓度的增加,AlCl 3的溶解度从31.20降低到0.13%,且AlCl 3 ·6H的结晶区2 O逐渐增加。ħ +浓度对的FeCl影响不大3的溶解度,和的FeCl的溶解度3是47.52%。通过理论计算,可以通过由不同材料组成的系统的计算模型获得所需的水分蒸发和晶体沉淀。文献中通过相平衡和相图理论,从煤石的铝酸浸出液中分离铝和铁,设计了路线。通过蒸发和结晶得到的AlCl 3 ·6H 2 O的直接产率为77.15%,用浓盐酸冲洗后纯度达到99.81%。
更新日期:2019-11-11
中文翻译:

AlCl 3 -FeCl 3 -H 2 O(-HCl)盐水系统在298.15 K时的相图及其在AlCl 3 ·6H 2 O结晶中的应用
铝铁分离在湿法冶金领域引起了极大的兴趣。使用相平衡理论,分步结晶和分离混合盐溶液是一种相对有效的方法。采用等温溶出平衡法研究了不同H +浓度下三元体系或三元体系AlCl 3 -FeCl 3 -H 2 O(-HCl)在298.15 K时的相平衡,并绘制了相应的相图和密度图。实验结果表明,随着H +浓度的增加,AlCl 3的溶解度从31.20降低到0.13%,且AlCl 3 ·6H的结晶区2 O逐渐增加。ħ +浓度对的FeCl影响不大3的溶解度,和的FeCl的溶解度3是47.52%。通过理论计算,可以通过由不同材料组成的系统的计算模型获得所需的水分蒸发和晶体沉淀。文献中通过相平衡和相图理论,从煤石的铝酸浸出液中分离铝和铁,设计了路线。通过蒸发和结晶得到的AlCl 3 ·6H 2 O的直接产率为77.15%,用浓盐酸冲洗后纯度达到99.81%。













































 京公网安备 11010802027423号
京公网安备 11010802027423号