当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cation Channel Transient Receptor Potential Vanilloid 4 Mediates Topography-Induced Osteoblastic Differentiation of Bone Marrow Stem Cells
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2019-11-22 , DOI: 10.1021/acsbiomaterials.9b01237 Wenqing Hou , Hong Fu , Xuan Liu , Ke Duan , Xiaobo Lu , Mengjie Lu , Tong Sun , Tailin Guo , Jie Weng
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2019-11-22 , DOI: 10.1021/acsbiomaterials.9b01237 Wenqing Hou , Hong Fu , Xuan Liu , Ke Duan , Xiaobo Lu , Mengjie Lu , Tong Sun , Tailin Guo , Jie Weng
|
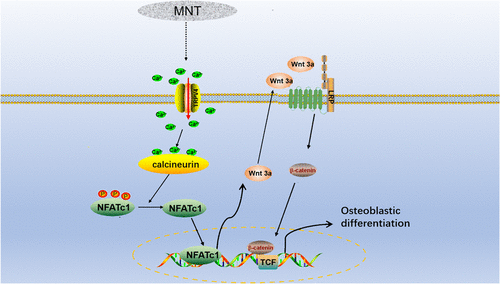
Micro/nanotopographies (MNTs) have been reported to enhance the osseointegration of biomaterials and modulate cell functions, but the underlying mechanisms are incompletely understood. We hypothesized that transient receptor potential vanilloid 4 (TRPV4) may mediate the topographically induced osteoblastic differentiation of bone marrow stem cells (BMSCs) by regulating the NFATc1 and Wnt/β-catenin signaling. To test this hypothesis, murine BMSCs were cultured on polished titanium (Ti) discs (PT) and Ti discs carrying titania nanotubes (i.e., MNTs) with diameters of ∼30 and ∼100 nm (termed TNT-30 and TNT-100, respectively). It was found that the MNTs (in particular TNT-100) promoted the expression and activation of TRPV4. Inhibition of TRPV4 in BMSCs cultured on TNT-100 reduced the expression of osteoblastic genes and the gene expression and protein levels of NFATc1 and Wnt3a/β-catenin and also decreased nuclear translocation of NFATc1 and β-catenin (all vs uninhibited BMSCs). Conversely, activation of TRPV4 in BMSCs cultured on PT increased the expression of the osteoblastic gene and the gene expression and protein level of NFATc1 and Wnt3a/β-catenin and also enhanced the nuclear translocation of NFATc1 and β-catenin (all vs unactivated BMSCs). These differences suggest that the MNTs promoted TRPV4 expression and activation to enhance intracellular Ca2+, which further increased the nuclear translocation of NFATc1 and stimulated the Wnt/β-catenin signaling, thus leading to upregulated expression of osteoblastic genes. These results indicate TRPV4 to be a mediator in MNT-induced osteoblastic differentiation of BMSCs.
中文翻译:

阳离子通道瞬态受体电位香草酸4介导的地形诱导骨髓干细胞的成骨细胞分化。
微型/纳米地形学(MNTs)已被报道可以增强生物材料的骨整合并调节细胞功能,但其潜在机理尚未完全了解。我们假设瞬时受体电位香草酸4(TRPV4)可能通过调节NFATc1和Wnt /β-catenin信号传导介导了地形诱导的骨髓干细胞(BMSCs)的成骨细胞分化。为了验证这一假设,将鼠骨髓间充质干细胞培养在直径为30纳米和100纳米(分别称为TNT-30和TNT-100)的带有氧化钛纳米管(即MNT)的抛光钛(Ti)圆盘(PT)和Ti圆盘上。 )。发现MNT(特别是TNT-100)促进了TRPV4的表达和激活。在TNT-100上培养的BMSC中TRPV4的抑制降低了成骨细胞基因的表达以及NFATc1和Wnt3a /β-catenin的基因表达和蛋白质水平,并且还减少了NFATc1和β-catenin的核易位(所有与未抑制的BMSC相比)。相反,在PT上培养的BMSC中TRPV4的激活增加了成骨细胞基因的表达以及NFATc1和Wnt3a /β-catenin的基因表达和蛋白质水平,还增强了NFATc1和β-catenin的核转运(所有与未激活的BMSC相比) 。这些差异表明,MNTs促进了TRPV4的表达和激活,从而增强了细胞内Ca的表达。在PT上培养的BMSC中TRPV4的激活增加了成骨细胞基因的表达以及NFATc1和Wnt3a /β-catenin的基因表达和蛋白质水平,并且还增强了NFATc1和β-catenin的核易位(所有与未激活的BMSC相比)。这些差异表明,MNTs促进了TRPV4的表达和激活,从而增强了细胞内Ca的表达。在PT上培养的BMSC中TRPV4的激活增加了成骨细胞基因的表达以及NFATc1和Wnt3a /β-catenin的基因表达和蛋白质水平,并且还增强了NFATc1和β-catenin的核易位(所有与未激活的BMSC相比)。这些差异表明,MNTs促进了TRPV4的表达和激活,从而增强了细胞内Ca的表达。2+进一步增加NFATc1的核易位并刺激Wnt /β-catenin信号传导,从而导致成骨细胞基因表达上调。这些结果表明TRPV4是MNT诱导的BMSCs成骨细胞分化的介体。
更新日期:2019-11-22
中文翻译:

阳离子通道瞬态受体电位香草酸4介导的地形诱导骨髓干细胞的成骨细胞分化。
微型/纳米地形学(MNTs)已被报道可以增强生物材料的骨整合并调节细胞功能,但其潜在机理尚未完全了解。我们假设瞬时受体电位香草酸4(TRPV4)可能通过调节NFATc1和Wnt /β-catenin信号传导介导了地形诱导的骨髓干细胞(BMSCs)的成骨细胞分化。为了验证这一假设,将鼠骨髓间充质干细胞培养在直径为30纳米和100纳米(分别称为TNT-30和TNT-100)的带有氧化钛纳米管(即MNT)的抛光钛(Ti)圆盘(PT)和Ti圆盘上。 )。发现MNT(特别是TNT-100)促进了TRPV4的表达和激活。在TNT-100上培养的BMSC中TRPV4的抑制降低了成骨细胞基因的表达以及NFATc1和Wnt3a /β-catenin的基因表达和蛋白质水平,并且还减少了NFATc1和β-catenin的核易位(所有与未抑制的BMSC相比)。相反,在PT上培养的BMSC中TRPV4的激活增加了成骨细胞基因的表达以及NFATc1和Wnt3a /β-catenin的基因表达和蛋白质水平,还增强了NFATc1和β-catenin的核转运(所有与未激活的BMSC相比) 。这些差异表明,MNTs促进了TRPV4的表达和激活,从而增强了细胞内Ca的表达。在PT上培养的BMSC中TRPV4的激活增加了成骨细胞基因的表达以及NFATc1和Wnt3a /β-catenin的基因表达和蛋白质水平,并且还增强了NFATc1和β-catenin的核易位(所有与未激活的BMSC相比)。这些差异表明,MNTs促进了TRPV4的表达和激活,从而增强了细胞内Ca的表达。在PT上培养的BMSC中TRPV4的激活增加了成骨细胞基因的表达以及NFATc1和Wnt3a /β-catenin的基因表达和蛋白质水平,并且还增强了NFATc1和β-catenin的核易位(所有与未激活的BMSC相比)。这些差异表明,MNTs促进了TRPV4的表达和激活,从而增强了细胞内Ca的表达。2+进一步增加NFATc1的核易位并刺激Wnt /β-catenin信号传导,从而导致成骨细胞基因表达上调。这些结果表明TRPV4是MNT诱导的BMSCs成骨细胞分化的介体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号