Life Sciences ( IF 5.2 ) Pub Date : 2019-07-11 , DOI: 10.1016/j.lfs.2019.116642 Kaili Wang 1 , Danxia Chu 2 , Jie Wu 2 , Mengling Zhao 2 , Miaomiao Zhang 2 , Bijun Li 2 , Wenjun Du 2 , Jianmin Du 2 , Ruixia Guo 2
|
Purpose
Cinobufagin(CB), an cardiotonic steroid isolated from the skin and parotid venom glands of the toad Bufo bufo gargarizans Cantor, has reported to have a significant anti-cancer effect on various cancers. However, the effect of CB on ovarian cancers was none reported. Herein, the present study aimed to investigate the therapeutic effect of cinobufagin on the ovarian cancer cells and elucidate the underlying mechanism.
Methods
Cell viability in our work was assessed via MTT. Cell apoptosis was detected by flow cytometry analysis and Hoechst 33258. Autophagy was defined by confocal microscopy after infected with mRFP-GFP-LC3 dual fluorescence adenovirus. Reactive oxygen species (ROS) was investigated by flow cytometry. The level of marker proteins involved in autophagy, apoptosis and ROS/MAPK signaling pathway were determined by western blot.
Results
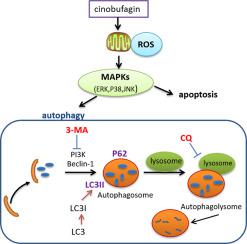
Cinobufagin significantly reduced the viability and induced apoptotic cell death of human ovarian cancer cell lines SKOV-3 and A2780. MRFP-GFP-LC3 infection elaborated that cinobufagin could promote cell autophagy. Moreover, autophagy inhibitor 3-methyladenine (3-MA) markedly enhanced the cinobufagin-induced apoptosis. In addition, treatment with cinobufagin could dramatically increase the expression of ROS and then activate the phosphorylation of MAPK family proteins, including ERK 1/2, JNK and p38. What's more, the reaction of apoptosis and autophagy induced by cinobufagin treatment could be reversed by p38 inhibitor SB203580 and JNK inhibitor SP600125 as well as ROS exclusive inhibitor antioxidant N-acetyl cysteine (NAC).
Conclusions
Our findings provide clues concluding that cinobufagin could induce cell apoptosis and protective autophagy through the ROS/MAPK signaling pathway in human ovarian cancer cells.
中文翻译:

撤回:华蟾素通过 ROS/MAPK 信号通路诱导细胞凋亡和保护性自噬。
目的
华蟾素 (CB) 是一种从蟾蜍Bufo bufo gargarizans Cantor 的皮肤和腮腺毒腺中分离出来的强心类固醇,据报道对多种癌症具有显着的抗癌作用。然而,CB 对卵巢癌的作用尚未见报道。本研究旨在探讨华蟾素对卵巢癌细胞的治疗作用并阐明其潜在机制。
方法
我们工作中的细胞活力通过 MTT 进行评估。通过流式细胞仪分析和Hoechst 33258检测细胞凋亡。用mRFP-GFP-LC3双荧光腺病毒感染后,通过共聚焦显微镜定义自噬。通过流式细胞术研究活性氧(ROS)。通过蛋白质印迹法测定参与自噬、凋亡和ROS/MAPK信号通路的标志蛋白的水平。
结果
华蟾素显着降低人卵巢癌细胞系 SKOV-3 和 A2780 的活力并诱导细胞凋亡。 MRFP-GFP-LC3感染阐明华蟾素可以促进细胞自噬。此外,自噬抑制剂3-甲基腺嘌呤(3-MA)显着增强华蟾素诱导的细胞凋亡。此外,华蟾素治疗可以显着增加ROS的表达,然后激活MAPK家族蛋白的磷酸化,包括ERK 1/2、JNK和p38。更重要的是,华蟾素治疗诱导的细胞凋亡和自噬反应可以被p38抑制剂SB203580和JNK抑制剂SP600125以及ROS独家抑制剂抗氧化剂N-乙酰半胱氨酸(NAC)逆转。
结论
我们的研究结果提供了线索,表明华蟾素可以通过人卵巢癌细胞中的 ROS/MAPK 信号通路诱导细胞凋亡和保护性自噬。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号