当前位置:
X-MOL 学术
›
Powder Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction mechanism study of new scheme using elemental sulfur for conversion of barite to barium sulfide
Powder Technology ( IF 4.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.powtec.2019.10.088 Wei Zhang , Fengzhen Zhang , Liping Ma , Jie Yang , Jing Yang , Huaping Xiang
Powder Technology ( IF 4.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.powtec.2019.10.088 Wei Zhang , Fengzhen Zhang , Liping Ma , Jie Yang , Jing Yang , Huaping Xiang
|
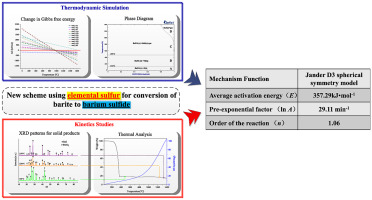
Abstract Barium sulfide has been widely accepted as a useful barium compound in many fields. To reduce the emission of CO2 through tail gas during the current industrial production of barium sulfide by the black ash method, a new clean process for barium sulfide preparation by barite reduction using elemental sulfur is proposed. Herein, we report a study on the kinetics and thermodynamics of the thermal decomposition of barium sulfate by elemental sulfur. The mechanism of this complicated gas–solid reaction was investigated under non-isothermal conditions, at different heating rates. Four isoconversional methods were used to determine the activation energy. The mechanism model of the barium sulfate decomposition was confirmed using the double equal–double step method. The results suggested that the barium sulfate decomposition followed the Jander D3 spherical symmetry model; the order of the reaction (n = 1.06) was determined using the Carne equation.
中文翻译:

单质硫重晶石转化硫化钡新方案的反应机理研究
摘要 硫化钡作为一种有用的钡化合物已被许多领域广泛接受。为减少目前黑灰法硫化钡工业生产过程中尾气CO2的排放,提出了一种利用元素硫重晶石还原制备硫化钡的清洁新工艺。在此,我们报告了硫酸钡被元素硫热分解的动力学和热力学研究。在非等温条件下,在不同的加热速率下研究了这种复杂的气固反应的机理。使用四种等转化方法来确定活化能。采用双等双步法确定了硫酸钡分解的机理模型。结果表明硫酸钡的分解遵循Jander D3球对称模型;使用 Carne 方程确定反应的顺序 (n = 1.06)。
更新日期:2020-01-01
中文翻译:

单质硫重晶石转化硫化钡新方案的反应机理研究
摘要 硫化钡作为一种有用的钡化合物已被许多领域广泛接受。为减少目前黑灰法硫化钡工业生产过程中尾气CO2的排放,提出了一种利用元素硫重晶石还原制备硫化钡的清洁新工艺。在此,我们报告了硫酸钡被元素硫热分解的动力学和热力学研究。在非等温条件下,在不同的加热速率下研究了这种复杂的气固反应的机理。使用四种等转化方法来确定活化能。采用双等双步法确定了硫酸钡分解的机理模型。结果表明硫酸钡的分解遵循Jander D3球对称模型;使用 Carne 方程确定反应的顺序 (n = 1.06)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号