当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Tolbutamide and Chlorpropamide in Supercritical Carbon Dioxide
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acs.jced.8b00050 Luigi Manna 1 , Mauro Banchero 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acs.jced.8b00050 Luigi Manna 1 , Mauro Banchero 1
Affiliation
|
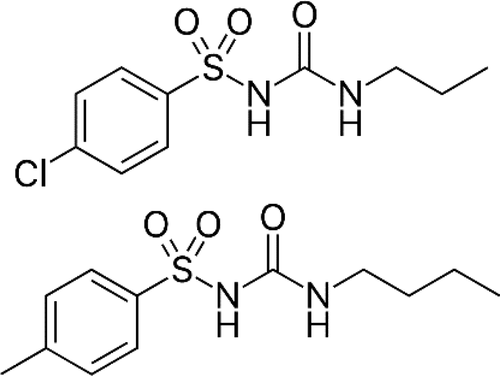
Tolbutamide and chlorpropamide are oral hypoglycemic drugs that are used to treat diabetic patients. In the context of employing these drugs in green pharmaceutical applications that make use of supercritical fluids, the solubility of tolbutamide and chlorpropamide in supercritical CO2 has been measured at 313.15, 333.15, and 353.15 K and in the pressure range of 10–30 MPa. A semiflow apparatus equipped with a continuous solvent-dilution device of the depressurization line, which avoids the solubility data to be underestimated, was employed. The solubility of tolbutamide is in the range of 1.66 × 10–5 to 40.5 × 10–5 mole fraction while that of chlorpropamide is in the range of 2.29 × 10–6 to 72.2 × 10–6 mole fraction, which indicates that the first drug has higher solubility in the supercritical fluid than the latter, probably due to its higher hydrophobicity. The results were successfully correlated with the most popular empirical and semiempirical models reported in the literature. The Sparks model provided the best correlation for chlorpropamide with an adjusted absolute average percent deviation (AARD%) of 3.5%, while the Keshmiri model provided the best correlation for tolbutamide, with an AARD% of 4.8%. The self-consistency of the experimental data was checked through the Méndez-Santiago and Teja model.
中文翻译:

甲苯磺丁酰胺和氯丙酰胺在超临界二氧化碳中的溶解度
甲苯磺丁酰胺和氯丙酰胺是用于治疗糖尿病患者的口服降糖药。在将这些药物用于使用超临界流体的绿色制药应用中,测得的甲苯磺丁酰胺和氯丙酰胺在超临界CO 2中的溶解度为313.15、333.15和353.15 K,压力范围为10-30 MPa。使用一种配备有减压管线的连续溶剂稀释装置的半流设备,该设备避免了溶解度数据被低估。甲苯磺丁酰胺的溶解度在1.66×10 –5至40.5×10 –5摩尔分数的范围内,而氯丙酰胺的溶解度在2.29×10 –6至72.2×10 –6的范围内摩尔分数,这表明第一种药物在超临界流体中的溶解度高于后者,可能是由于其较高的疏水性。结果成功地与文献中报道的最流行的经验和半经验模型相关。Sparks模型为氯丙酰胺提供了最佳相关性,调整后的绝对平均百分偏差(AARD%)为3.5%,而Keshmiri模型为甲苯磺丁酰胺提供了最佳相关性,AARD%为4.8%。通过Méndez-Santiago和Teja模型检查了实验数据的自洽性。
更新日期:2018-03-10
中文翻译:

甲苯磺丁酰胺和氯丙酰胺在超临界二氧化碳中的溶解度
甲苯磺丁酰胺和氯丙酰胺是用于治疗糖尿病患者的口服降糖药。在将这些药物用于使用超临界流体的绿色制药应用中,测得的甲苯磺丁酰胺和氯丙酰胺在超临界CO 2中的溶解度为313.15、333.15和353.15 K,压力范围为10-30 MPa。使用一种配备有减压管线的连续溶剂稀释装置的半流设备,该设备避免了溶解度数据被低估。甲苯磺丁酰胺的溶解度在1.66×10 –5至40.5×10 –5摩尔分数的范围内,而氯丙酰胺的溶解度在2.29×10 –6至72.2×10 –6的范围内摩尔分数,这表明第一种药物在超临界流体中的溶解度高于后者,可能是由于其较高的疏水性。结果成功地与文献中报道的最流行的经验和半经验模型相关。Sparks模型为氯丙酰胺提供了最佳相关性,调整后的绝对平均百分偏差(AARD%)为3.5%,而Keshmiri模型为甲苯磺丁酰胺提供了最佳相关性,AARD%为4.8%。通过Méndez-Santiago和Teja模型检查了实验数据的自洽性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号