当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quinoidal Oligo(9,10‐anthryl)s with Chain‐Length‐Dependent Ground States: A Balance between Aromatic Stabilization and Steric Strain Release
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-11-12 , DOI: 10.1002/chem.201503033 Zhenglong Lim , Bin Zheng , Kuo-Wei Huang , Ye Liu , Jishan Wu
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-11-12 , DOI: 10.1002/chem.201503033 Zhenglong Lim , Bin Zheng , Kuo-Wei Huang , Ye Liu , Jishan Wu
|
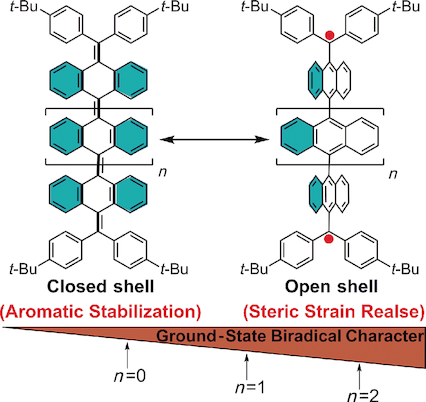
Quinoidal π‐conjugated polycyclic hydrocarbons have attracted intensive research interest due to their unique optical/electronic properties and possible magnetic activity, which arises from a thermally excited triplet state. However, there is still lack of fundamental understanding on the factors that determine the electronic ground states. Herein, by using quinoidal oligo(9,10‐anthryl)s, it is demonstrated that both aromatic stabilisation and steric strain release play balanced roles in determining the ground states. Oligomers with up to four anthryl units were synthesised and their ground states were investigated by electronic absorption and electron spin resonance (ESR) spectroscopy, assisted by density functional theory (DFT) calculations. The quinoidal 9,10‐anthryl dimer 1 has a closed‐shell ground state, whereas the tri‐ (2) and tetramers (3) both have an open‐shell diradical ground state with a small singlet–triplet gap. Such a difference results from competition between two driving forces: the large steric repulsion between the anthryl/phenyl units in the closed‐shell quinoidal form that drives the molecule to a flexible open‐shell diradical structure, and aromatic stabilisation due to the gain of more aromatic sextet rings in the closed‐shell form, which drives the molecule towards a contorted quinoidal structure. The ground states of these oligomers thus depend on the overall balance between these two driving forces and show chain‐length dependence.
中文翻译:

链长依赖基态的Quinoidal Oligo(9,10-anthryl)s:芳香稳定和释放立体应变之间的平衡。
醌型π共轭多环烃因其独特的光学/电子特性和可能的磁活性而引起了广泛的研究兴趣,这可能是由热激发的三重态引起的。但是,对于确定电子基态的因素仍然缺乏基本的了解。本文中,通过使用醌型寡聚(9,10-蒽基),芳族稳定和空间应变释放在确定基态中均起平衡作用。合成了最多具有四个蒽基的低聚物,并借助电子吸收和电子自旋共振(ESR)光谱,并借助密度泛函理论(DFT)计算,研究了它们的基态。quinoidal 9,10-蒽二聚体1具有闭壳基态,而三聚体(2)和四聚体(3)均具有开壳双自由基基态,且单线态-三重态间隙较小。产生这种差异的原因是两种驱动力之间的竞争:闭壳喹啉形式的蒽基/苯基单元之间的大空间排斥将分子驱动为柔性的开壳双自由基结构,而芳香族稳定化则得益于更多的获得。闭环形式的芳族六重环,将分子推向扭曲的喹啉结构。因此,这些低聚物的基态取决于这两个驱动力之间的总体平衡,并显示出链长依赖性。
更新日期:2015-11-12
中文翻译:

链长依赖基态的Quinoidal Oligo(9,10-anthryl)s:芳香稳定和释放立体应变之间的平衡。
醌型π共轭多环烃因其独特的光学/电子特性和可能的磁活性而引起了广泛的研究兴趣,这可能是由热激发的三重态引起的。但是,对于确定电子基态的因素仍然缺乏基本的了解。本文中,通过使用醌型寡聚(9,10-蒽基),芳族稳定和空间应变释放在确定基态中均起平衡作用。合成了最多具有四个蒽基的低聚物,并借助电子吸收和电子自旋共振(ESR)光谱,并借助密度泛函理论(DFT)计算,研究了它们的基态。quinoidal 9,10-蒽二聚体1具有闭壳基态,而三聚体(2)和四聚体(3)均具有开壳双自由基基态,且单线态-三重态间隙较小。产生这种差异的原因是两种驱动力之间的竞争:闭壳喹啉形式的蒽基/苯基单元之间的大空间排斥将分子驱动为柔性的开壳双自由基结构,而芳香族稳定化则得益于更多的获得。闭环形式的芳族六重环,将分子推向扭曲的喹啉结构。因此,这些低聚物的基态取决于这两个驱动力之间的总体平衡,并显示出链长依赖性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号