当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of Micromonocyclol Synthase from the Marine Actinomycete Micromonospora marina.
Organic Letters ( IF 4.9 ) Pub Date : 2019-11-08 , DOI: 10.1021/acs.orglett.9b03654 Jan Rinkel 1 , Jeroen S Dickschat 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-11-08 , DOI: 10.1021/acs.orglett.9b03654 Jan Rinkel 1 , Jeroen S Dickschat 1
Affiliation
|
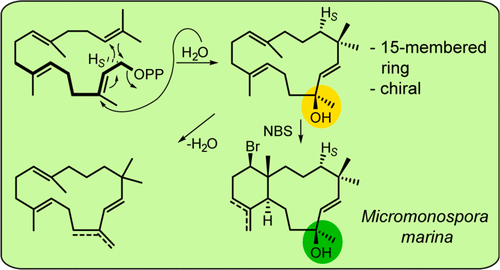
As a member of a large phylogenetic clade of enzymes in Micromonospora, a terpene synthase from M. marina is functionally characterized to produce micromonocyclol. This diterpene alcohol features a rare 15-membered ring, which prevented elucidation of the only stereocenter by labeling experiments. This problem was addressed by chemical transformation into bicyclic brominated derivatives, whose rigidified skeletons allowed for a stereochemical assignment. Using this strategy, a complete stereochemical model of the cyclization mechanism was also elaborated.
中文翻译:

从海洋放线菌Micromonospora码头的微单环合酶的表征。
作为微单孢菌中较大的系统进化酶成员,来自滨海分枝杆菌的萜烯合酶在功能上具有产生微单环的特征。这种二萜醇具有罕见的15元环,可通过标记实验阻止阐明唯一的立体中心。通过化学转化为双环溴化衍生物解决了该问题,该衍生物的刚性骨架可以实现立体化学分配。使用该策略,还阐述了环化机理的完整立体化学模型。
更新日期:2019-11-08
中文翻译:

从海洋放线菌Micromonospora码头的微单环合酶的表征。
作为微单孢菌中较大的系统进化酶成员,来自滨海分枝杆菌的萜烯合酶在功能上具有产生微单环的特征。这种二萜醇具有罕见的15元环,可通过标记实验阻止阐明唯一的立体中心。通过化学转化为双环溴化衍生物解决了该问题,该衍生物的刚性骨架可以实现立体化学分配。使用该策略,还阐述了环化机理的完整立体化学模型。






































 京公网安备 11010802027423号
京公网安备 11010802027423号