Communications Chemistry ( IF 5.9 ) Pub Date : 2019-11-07 , DOI: 10.1038/s42004-019-0224-2 Jos J. A. G. Kamps , Richard J. Hopkinson , Christopher J. Schofield , Timothy D. W. Claridge
|
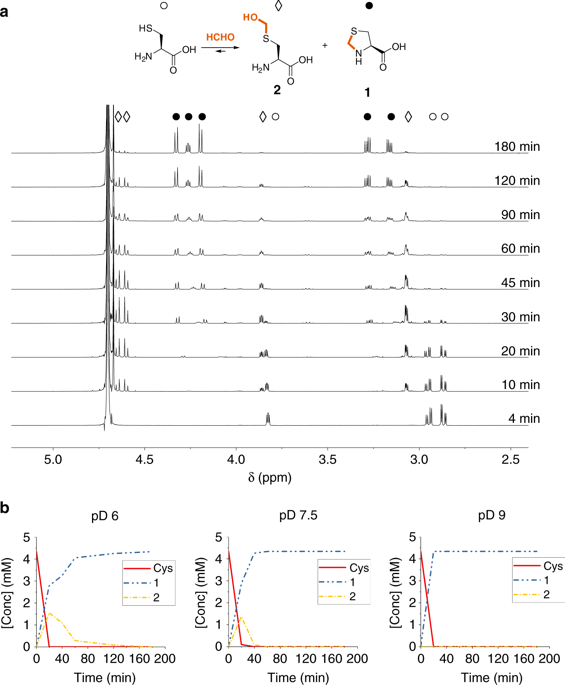
Formaldehyde is a biological electrophile produced via processes including enzymatic demethylation. Despite its apparent simplicity, the reactions of formaldehyde with even basic biological components are incompletely defined. Here we report NMR-based studies on the reactions of formaldehyde with common proteinogenic and other nucleophilic amino acids. The results reveal formaldehyde reacts at different rates, forming hydroxymethylated, cyclised, cross-linked, or disproportionated products of varying stabilities. Of the tested common amino acids, cysteine reacts most efficiently, forming a stable thiazolidine. The reaction with lysine is less efficient; low levels of an Nε-methylated product are observed, raising the possibility of non-enzymatic lysine methylation by formaldehyde. Reactions with formaldehyde are faster than reactions with other tested biological carbonyl compounds, and the adducts are also more stable. The results reveal reactions of formaldehyde with amino acids, and by extension peptides and proteins, have potential roles in healthy and diseased biology, as well as in evolution.
中文翻译:

甲醛如何与氨基酸反应
甲醛是一种通过包括酶促脱甲基在内的过程产生的生物亲电试剂。尽管它看起来很简单,但甲醛与基本生物成分的反应仍未完全确定。在这里,我们报告基于NMR的甲醛与常见蛋白原和其他亲核氨基酸反应的研究。结果表明甲醛以不同的速率反应,形成具有不同稳定性的羟甲基化,环化,交联或歧化的产物。在测试的常见氨基酸中,半胱氨酸最有效地反应,形成稳定的噻唑烷。与赖氨酸的反应效率较低。低水平的Nε观察到甲基化产物,增加了甲醛进行非酶赖氨酸甲基化的可能性。与甲醛的反应比与其他经过测试的生物羰基化合物的反应要快,并且加合物也更稳定。结果显示甲醛与氨基酸的反应,以及延伸肽和蛋白质的反应,在健康和患病的生物学以及进化中具有潜在作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号