当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evidence of C-H···O Interactions in the Thiophene:Water Complex.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-11-20 , DOI: 10.1021/acs.jpca.9b07355
Joshua G Wasserman 1 , Keshihito J Murphy 1 , Josh J Newby 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-11-20 , DOI: 10.1021/acs.jpca.9b07355
Joshua G Wasserman 1 , Keshihito J Murphy 1 , Josh J Newby 1
Affiliation
|
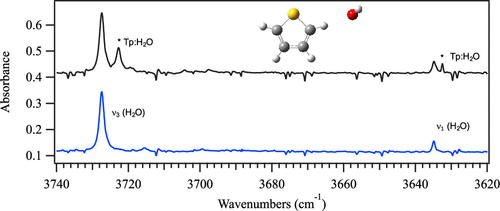
An analysis of the 1:1 complex of thiophene and water is presented. In this study, computation and matrix isolation Fourier transform infrared spectroscopy (FTIR) were used to determine stable complexes of thiophene and water. Computational studies found six, low-energy complexes that were differentiated by the interaction present. Three complexes were characterized by C-H···O interactions, one by O-H···S, one by O-H···π, and one with an unusual, dual interaction of O-H···S and C-H···O. The O-H···π interaction was found to have the lowest overall energy at multiple levels of theory (B3LYP, B3LYP-GD3BJ, B97-D3, M05-2X, ωB97X-D, and MP2). Analysis of matrix isolation FTIR spectra indicated that the primary experimental geometry was a complex where water interacts through C-H···O at the α carbon position of the thiophene ring. This experimental result is not in line with other related complexes (furan:water and thiophene:methanol) where the complex formed through more standard interactions (e.g., O-H···O and O-H···π). These atypical differences are explored in our findings.
中文翻译:

噻吩:水络合物中CH··O相互作用的证据。
分析了噻吩和水的1:1配合物。在这项研究中,计算和矩阵隔离傅里叶变换红外光谱(FTIR)用于确定噻吩和水的稳定络合物。计算研究发现了六种低能复合物,它们通过当前的相互作用得以区分。三种配合物的特征是CH···O相互作用,一种通过OH···S相互作用,一种通过OH···π相互作用,一种具有OH···S和CH···O异常的双重相互作用。在多个理论水平上(B3LYP,B3LYP-GD3BJ,B97-D3,M05-2X,ωB97X-D和MP2),发现OH··π相互作用的最低总能量。基质分离FTIR光谱分析表明,主要的实验几何结构是复杂的,水在噻吩环的α碳位置通过CH···O相互作用。该实验结果与其他相关的配合物(呋喃:水和噻吩:甲醇)不符,后者通过更标准的相互作用(例如OH··O和OH···π)形成了该配合物。在我们的发现中探索了这些非典型差异。
更新日期:2019-11-21
中文翻译:

噻吩:水络合物中CH··O相互作用的证据。
分析了噻吩和水的1:1配合物。在这项研究中,计算和矩阵隔离傅里叶变换红外光谱(FTIR)用于确定噻吩和水的稳定络合物。计算研究发现了六种低能复合物,它们通过当前的相互作用得以区分。三种配合物的特征是CH···O相互作用,一种通过OH···S相互作用,一种通过OH···π相互作用,一种具有OH···S和CH···O异常的双重相互作用。在多个理论水平上(B3LYP,B3LYP-GD3BJ,B97-D3,M05-2X,ωB97X-D和MP2),发现OH··π相互作用的最低总能量。基质分离FTIR光谱分析表明,主要的实验几何结构是复杂的,水在噻吩环的α碳位置通过CH···O相互作用。该实验结果与其他相关的配合物(呋喃:水和噻吩:甲醇)不符,后者通过更标准的相互作用(例如OH··O和OH···π)形成了该配合物。在我们的发现中探索了这些非典型差异。































 京公网安备 11010802027423号
京公网安备 11010802027423号