当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DNA framework-programmed cell capture via topology-engineered receptor-ligand interactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-11-06 , DOI: 10.1021/jacs.9b11015 Min Li 1 , Hongming Ding 2 , Meihua Lin 3 , Fangfei Yin 1, 4 , Lu Song 1, 4 , Xiuhai Mao 1 , Fan Li 1 , Zhilei Ge 1 , Lihua Wang 4 , Xiaolei Zuo 1 , Yuqiang Ma 5 , Chunhai Fan 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-11-06 , DOI: 10.1021/jacs.9b11015 Min Li 1 , Hongming Ding 2 , Meihua Lin 3 , Fangfei Yin 1, 4 , Lu Song 1, 4 , Xiuhai Mao 1 , Fan Li 1 , Zhilei Ge 1 , Lihua Wang 4 , Xiaolei Zuo 1 , Yuqiang Ma 5 , Chunhai Fan 1
Affiliation
|
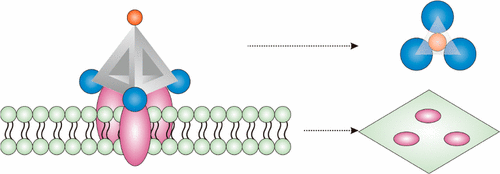
Receptor-ligand interactions (RLIs) that play pivotal roles in living organisms are often depicted with the classic keys-and-locks model. Nevertheless, RLIs on the cell surface are generally highly complex and nonlinear, partially due to the non-continuous and dynamic distribution of receptors on extracellular membranes. Here, we develop a tetrahedral DNA framework (TDF)-programmed approach to topologically engineer RLIs on the cell membrane, which enables active re-cruitment-binding of clustered receptors for high-affinity capture of circulating tumor cells (CTCs). The four vertices of a TDF afford orthogonal anchoring of ligands with spatial organization, based on which we synthesized n-simplexes har-boring 1-3 aptamers targeting epithelial cell adhesion molecule (EpCAM) that are over-expressed on the membrane of tumor cells. The 2-simplex with three aptamers not only shows increased binding affinity (~19-fold) but prevents endo-cytosis by cells. By using 2-simplex as the capture probe, we demonstrate the high-efficiency CTC capture, which is chal-lenged in real clinical breast cancer patient samples. This TDF-programmed platform thus provides a powerful means for studying RLIs in physiological settings and for cancer diagnosis.
中文翻译:

通过拓扑工程受体-配体相互作用进行 DNA 框架编程的细胞捕获
在生物体中发挥关键作用的受体-配体相互作用 (RLI) 通常用经典的钥匙锁模型来描述。然而,细胞表面的 RLI 通常高度复杂和非线性,部分原因是细胞外膜上受体的非连续和动态分布。在这里,我们开发了一种四面体 DNA 框架 (TDF) 编程方法,在细胞膜上对 RLI 进行拓扑工程设计,从而能够主动重新招募成簇受体,以高亲和力捕获循环肿瘤细胞 (CTC)。TDF 的四个顶点提供了具有空间组织的配体的正交锚定,在此基础上我们合成了 n-单纯形,包含 1-3 个靶向上皮细胞粘附分子 (EpCAM) 的适体,这些适体在肿瘤细胞膜上过表达。具有三个适体的 2-simplex 不仅显示出增加的结合亲和力(~19 倍),而且防止细胞的内吞作用。通过使用 2-simplex 作为捕获探针,我们展示了高效的 CTC 捕获,这在真实的临床乳腺癌患者样本中受到了挑战。因此,该 TDF 编程平台为研究生理环境中的 RLI 和癌症诊断提供了强大的手段。
更新日期:2019-11-06
中文翻译:

通过拓扑工程受体-配体相互作用进行 DNA 框架编程的细胞捕获
在生物体中发挥关键作用的受体-配体相互作用 (RLI) 通常用经典的钥匙锁模型来描述。然而,细胞表面的 RLI 通常高度复杂和非线性,部分原因是细胞外膜上受体的非连续和动态分布。在这里,我们开发了一种四面体 DNA 框架 (TDF) 编程方法,在细胞膜上对 RLI 进行拓扑工程设计,从而能够主动重新招募成簇受体,以高亲和力捕获循环肿瘤细胞 (CTC)。TDF 的四个顶点提供了具有空间组织的配体的正交锚定,在此基础上我们合成了 n-单纯形,包含 1-3 个靶向上皮细胞粘附分子 (EpCAM) 的适体,这些适体在肿瘤细胞膜上过表达。具有三个适体的 2-simplex 不仅显示出增加的结合亲和力(~19 倍),而且防止细胞的内吞作用。通过使用 2-simplex 作为捕获探针,我们展示了高效的 CTC 捕获,这在真实的临床乳腺癌患者样本中受到了挑战。因此,该 TDF 编程平台为研究生理环境中的 RLI 和癌症诊断提供了强大的手段。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号