当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isonitrile-responsive and bioorthogonally removable tetrazine protecting groups.
Chemical Science ( IF 7.6 ) Pub Date : 2019-11-05 , DOI: 10.1039/c9sc04649f Julian Tu 1 , Dennis Svatunek 2 , Saba Parvez 3 , Hannah J Eckvahl 2 , Minghao Xu 1 , Randall T Peterson 3 , K N Houk 2 , Raphael M Franzini 1
Chemical Science ( IF 7.6 ) Pub Date : 2019-11-05 , DOI: 10.1039/c9sc04649f Julian Tu 1 , Dennis Svatunek 2 , Saba Parvez 3 , Hannah J Eckvahl 2 , Minghao Xu 1 , Randall T Peterson 3 , K N Houk 2 , Raphael M Franzini 1
Affiliation
|
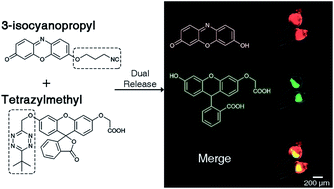
In vivo compatible reactions have a broad range of possible applications in chemical biology and the pharmaceutical sciences. Here we report tetrazines that can be removed by exposure to isonitriles under very mild conditions. Tetrazylmethyl derivatives are easily accessible protecting groups for amines and phenols. The isonitrile-induced removal is rapid and near-quantitative. Intriguingly, the deprotection is especially effective with (trimethylsilyl)methyl isocyanide, and serum albumin can catalyze the elimination under physiological conditions. NMR and computational studies revealed that an imine-tautomerization step is often rate limiting, and the unexpected cleavage of the Si-C bond accelerates this step in the case with (trimethylsilyl)methyl isocyanide. Tetrazylmethyl-removal is compatible with use on biomacromolecules, in cellular environments, and in living organisms as demonstrated by cytotoxicity experiments and fluorophore-release studies on proteins and in zebrafish embryos. By combining tetrazylmethyl derivatives with previously reported tetrazine-responsive 3-isocyanopropyl groups, it was possible to liberate two fluorophores in vertebrates from a single bioorthogonal reaction. This chemistry will open new opportunities towards applications involving multiplexed release schemes and is a valuable asset to the growing toolbox of bioorthogonal dissociative reactions.
中文翻译:

异腈响应且可生物正交去除的四嗪保护基团。
体内相容反应在化学生物学和制药科学中具有广泛的可能应用。在这里,我们报道了可以通过在非常温和的条件下接触异腈来去除四嗪。四嗪甲基衍生物是胺和酚的容易获得的保护基。异腈诱导的去除是快速且接近定量的。有趣的是,去保护对于(三甲基甲硅烷基)甲基异氰化物特别有效,并且血清白蛋白可以在生理条件下催化消除。 NMR 和计算研究表明,亚胺互变异构步骤通常是速率限制的,并且在使用(三甲基甲硅烷基)甲基异氰化物的情况下,Si-C 键的意外断裂加速了该步骤。正如细胞毒性实验以及蛋白质和斑马鱼胚胎中的荧光团释放研究所证明的那样,四甲基去除与生物大分子、细胞环境和活有机体中的使用兼容。通过将四嗪甲基衍生物与先前报道的四嗪响应性 3-异氰基丙基结合,可以从单个生物正交反应中释放脊椎动物中的两种荧光团。这种化学将为涉及多重释放方案的应用开辟新的机会,并且对于不断增长的生物正交解离反应工具箱来说是宝贵的资产。
更新日期:2019-12-19
中文翻译:

异腈响应且可生物正交去除的四嗪保护基团。
体内相容反应在化学生物学和制药科学中具有广泛的可能应用。在这里,我们报道了可以通过在非常温和的条件下接触异腈来去除四嗪。四嗪甲基衍生物是胺和酚的容易获得的保护基。异腈诱导的去除是快速且接近定量的。有趣的是,去保护对于(三甲基甲硅烷基)甲基异氰化物特别有效,并且血清白蛋白可以在生理条件下催化消除。 NMR 和计算研究表明,亚胺互变异构步骤通常是速率限制的,并且在使用(三甲基甲硅烷基)甲基异氰化物的情况下,Si-C 键的意外断裂加速了该步骤。正如细胞毒性实验以及蛋白质和斑马鱼胚胎中的荧光团释放研究所证明的那样,四甲基去除与生物大分子、细胞环境和活有机体中的使用兼容。通过将四嗪甲基衍生物与先前报道的四嗪响应性 3-异氰基丙基结合,可以从单个生物正交反应中释放脊椎动物中的两种荧光团。这种化学将为涉及多重释放方案的应用开辟新的机会,并且对于不断增长的生物正交解离反应工具箱来说是宝贵的资产。































 京公网安备 11010802027423号
京公网安备 11010802027423号