Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acetylation of the histone H3 tail domain regulates base excision repair on higher-order chromatin structures.
Scientific Reports ( IF 3.8 ) Pub Date : 2019-11-04 , DOI: 10.1038/s41598-019-52340-0 Deb Ranjan Banerjee 1 , Charles E Deckard 2 , Yu Zeng 3 , Jonathan T Sczepanski 2
Scientific Reports ( IF 3.8 ) Pub Date : 2019-11-04 , DOI: 10.1038/s41598-019-52340-0 Deb Ranjan Banerjee 1 , Charles E Deckard 2 , Yu Zeng 3 , Jonathan T Sczepanski 2
Affiliation
|
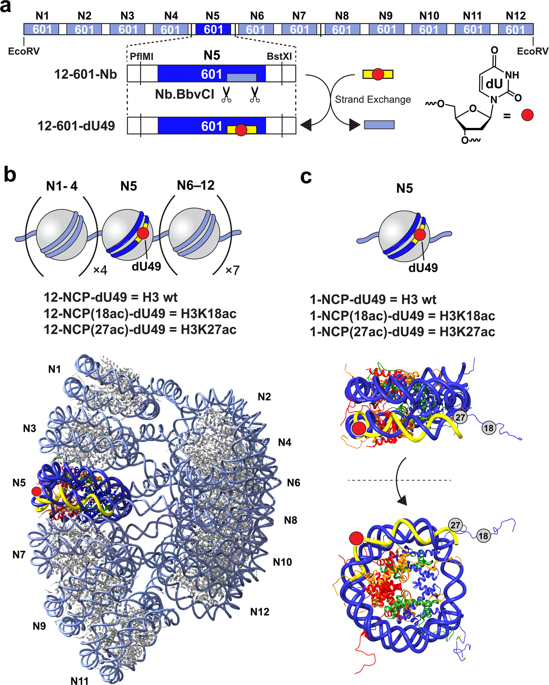
Despite recent evidence suggesting that histone lysine acetylation contributes to base excision repair (BER) in cells, their exact mechanistic role remains unclear. In order to examine the influence of histone acetylation on the initial steps of BER, we assembled nucleosome arrays consisting of homogeneously acetylated histone H3 (H3K18 and H3K27) and measured the repair of a site-specifically positioned 2'-deoxyuridine (dU) residue by uracil DNA glycosylase (UDG) and apurinic/apyrimidinic endonuclease 1 (APE1). We find that H3K18ac and H3K27ac differentially influence the combined activities of UDG/APE1 on compact chromatin, suggesting that acetylated lysine residues on the H3 tail domain play distinct roles in regulating the initial steps of BER. In addition, we show that the effects of H3 tail domain acetylation on UDG/APE1 activity are at the nucleosome level and do not influence higher-order chromatin folding. Overall, these results establish a novel regulatory role for histone H3 acetylation during the initiation of BER on chromatin.
中文翻译:

组蛋白H3尾部结构域的乙酰化调节了高级染色质结构上的碱基切除修复。
尽管最近的证据表明组蛋白赖氨酸乙酰化有助于细胞中的碱基切除修复(BER),但其确切的机械作用仍不清楚。为了检查组蛋白乙酰化对BER初始步骤的影响,我们组装了由均一乙酰化组蛋白H3(H3K18和H3K27)组成的核小体阵列,并测量了位点特异性定位的2'-脱氧尿苷(dU)残基的修复。尿嘧啶DNA糖基化酶(UDG)和嘌呤/嘧啶核糖核酸内切酶1(APE1)。我们发现H3K18ac和H3K27ac差异地影响UDG / APE1对紧凑染色质的联合活性,这表明H3尾部结构域上的乙酰化赖氨酸残基在调节BER的初始步骤中起着不同的作用。此外,我们表明,H3尾域乙酰化对UDG / APE1活性的影响是在核小体水平,并且不影响高阶染色质折叠。总体而言,这些结果为染色质上的BER引发过程中的组蛋白H3乙酰化建立了新的调节作用。
更新日期:2019-11-06
中文翻译:

组蛋白H3尾部结构域的乙酰化调节了高级染色质结构上的碱基切除修复。
尽管最近的证据表明组蛋白赖氨酸乙酰化有助于细胞中的碱基切除修复(BER),但其确切的机械作用仍不清楚。为了检查组蛋白乙酰化对BER初始步骤的影响,我们组装了由均一乙酰化组蛋白H3(H3K18和H3K27)组成的核小体阵列,并测量了位点特异性定位的2'-脱氧尿苷(dU)残基的修复。尿嘧啶DNA糖基化酶(UDG)和嘌呤/嘧啶核糖核酸内切酶1(APE1)。我们发现H3K18ac和H3K27ac差异地影响UDG / APE1对紧凑染色质的联合活性,这表明H3尾部结构域上的乙酰化赖氨酸残基在调节BER的初始步骤中起着不同的作用。此外,我们表明,H3尾域乙酰化对UDG / APE1活性的影响是在核小体水平,并且不影响高阶染色质折叠。总体而言,这些结果为染色质上的BER引发过程中的组蛋白H3乙酰化建立了新的调节作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号