Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo.
Nanoscale ( IF 5.8 ) Pub Date : 2019-12-04 , DOI: 10.1039/c9nr06505a Ke Li 1 , Lu Lu 1 , Chencheng Xue 1 , Ju Liu 1 , Ye He 1 , Jun Zhou 1 , Zengzilu Xia 1 , Liangliang Dai 1 , Zhong Luo 1 , Yulan Mao 1 , Kaiyong Cai 1
Nanoscale ( IF 5.8 ) Pub Date : 2019-12-04 , DOI: 10.1039/c9nr06505a Ke Li 1 , Lu Lu 1 , Chencheng Xue 1 , Ju Liu 1 , Ye He 1 , Jun Zhou 1 , Zengzilu Xia 1 , Liangliang Dai 1 , Zhong Luo 1 , Yulan Mao 1 , Kaiyong Cai 1
Affiliation
|
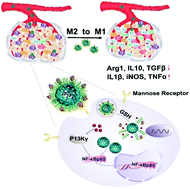
Tumor-associated macrophages (TAMs) are the most important components in the tumor immunosuppressive microenvironment, promoting tumor growth and metastasis. Although TAMs have become one of the hot topics of tumor immunotherapy, challenges still remain to achieve TAM-targeted re-polarization therapy. In this work, porous hollow iron oxide nanoparticles (PHNPs) were synthesized for loading a P13K γ small molecule inhibitor (3-methyladenine, 3-MA) and further modified by mannose to target TAMs. The delivery system named PHNPs@DPA-S-S-BSA-MA@3-MA showed good efficiency for targeting TAMs. The inflammatory factor NF-κB p65 of macrophages was activated by the combination of PHNPs and 3-MA, which synergistically switched TAMs to pro-inflammatory M1-type macrophages. As a result, it activated immune responses and inhibited tumor growth in vivo. The study provides an intracellular switch of the TAM phenotype for targeted TAM therapy.
中文翻译:

通过多孔空心铁纳米粒子极化与肿瘤相关的巨噬细胞表型,用于体内的肿瘤免疫治疗。
肿瘤相关巨噬细胞(TAM)是肿瘤免疫抑制微环境中最重要的组成部分,可促进肿瘤的生长和转移。尽管TAM已成为肿瘤免疫治疗的热门话题之一,但实现TAM靶向的重新极化治疗仍然面临挑战。在这项工作中,合成了多孔中空氧化铁纳米粒子(PHNPs)以装载P13Kγ小分子抑制剂(3-甲基腺嘌呤,3-MA),并进一步被甘露糖修饰以靶向TAM。名为PHNPs @ DPA-SS-BSA-MA @ 3-MA的传递系统显示出针对TAM的良好效率。PHNP和3-MA的结合可激活巨噬细胞的炎性因子NF-κBp65,从而将TAM协同转换为促炎性M1型巨噬细胞。结果,它在体内激活了免疫反应并抑制了肿瘤的生长。
更新日期:2019-12-19
中文翻译:

通过多孔空心铁纳米粒子极化与肿瘤相关的巨噬细胞表型,用于体内的肿瘤免疫治疗。
肿瘤相关巨噬细胞(TAM)是肿瘤免疫抑制微环境中最重要的组成部分,可促进肿瘤的生长和转移。尽管TAM已成为肿瘤免疫治疗的热门话题之一,但实现TAM靶向的重新极化治疗仍然面临挑战。在这项工作中,合成了多孔中空氧化铁纳米粒子(PHNPs)以装载P13Kγ小分子抑制剂(3-甲基腺嘌呤,3-MA),并进一步被甘露糖修饰以靶向TAM。名为PHNPs @ DPA-SS-BSA-MA @ 3-MA的传递系统显示出针对TAM的良好效率。PHNP和3-MA的结合可激活巨噬细胞的炎性因子NF-κBp65,从而将TAM协同转换为促炎性M1型巨噬细胞。结果,它在体内激活了免疫反应并抑制了肿瘤的生长。































 京公网安备 11010802027423号
京公网安备 11010802027423号