当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Addition of Isocyanide-Containing Amino Acids to the Genetic Code for Protein Labeling and Activation.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-11-14 , DOI: 10.1021/acschembio.9b00678
Yuda Chen 1 , Kuan-Lin Wu 1 , Juan Tang 1 , Axel Loredo 1 , Jordan Clements 1 , Jingqi Pei 2 , Zane Peng 2 , Ruchi Gupta 1 , Xinlei Fang 1 , Han Xiao 1, 2, 3
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-11-14 , DOI: 10.1021/acschembio.9b00678
Yuda Chen 1 , Kuan-Lin Wu 1 , Juan Tang 1 , Axel Loredo 1 , Jordan Clements 1 , Jingqi Pei 2 , Zane Peng 2 , Ruchi Gupta 1 , Xinlei Fang 1 , Han Xiao 1, 2, 3
Affiliation
|
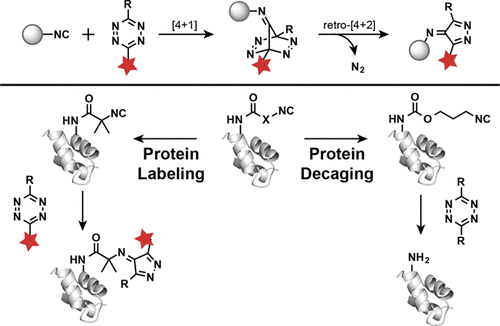
Site-specific introduction of bioorthogonal handles into biomolecules provides powerful tools for studying and manipulating the structures and functions of proteins. Recent advances in bioorthogonal chemistry demonstrate that tetrazine-based bioorthogonal cycloaddition is a particularly useful methodology due to its high reactivity, biological selectivity, and turn-on property for fluorescence imaging. Despite its broad applications in protein labeling and imaging, utilization of tetrazine-based bioorthogonal cycloaddition has been limited to date by the requirement of a hydrophobic strained alkene reactive moiety. Circumventing this structural requirement, we report the site-specific incorporation of noncanonical amino acids (ncAAs) with a small isocyanide (or isonitrile) group into proteins in both bacterial and mammalian cells. We showed that under physiological conditions and in the absence of a catalyst these isocyanide-containing ncAAs could react selectively with tetrazine molecules via [4 + 1]-cycloaddition, thus providing a versatile bioorthogonal handle for site-specific protein labeling and protein decaging. Significantly, these bioorthogonal reactions between isocyanides and tetrazines also provide a unique mechanism for the activation of tetrazine-quenched fluorophores. The addition of these isocyanide-containing ncAAs to the list of 20 commonly used, naturally occurring amino acids expands our repertoire of reagents for bioorthogonal chemistry, therefore enabling new biological applications ranging from protein labeling and imaging studies to the chemical activation of proteins.
中文翻译:

在蛋白质标记和激活的遗传密码中添加了含异氰酸酯的氨基酸。
在生物分子中特定位置引入生物正交手柄为研究和操纵蛋白质的结构和功能提供了强大的工具。生物正交化学的最新进展表明,基于四嗪的生物正交环加成因其高反应性,生物选择性和荧光成像的开启特性而成为一种特别有用的方法。尽管其在蛋白质标记和成像中具有广泛的应用,但由于疏水性应变烯烃反应性部分的要求,基于四嗪的生物正交环加成的应用迄今受到限制。绕过此结构要求,我们报告了非标准氨基酸(ncAAs)与小的异氰酸酯(或异腈)基团在细菌和哺乳动物细胞中的蛋白质中的位点特异性结合。我们表明,在生理条件下,在没有催化剂的情况下,这些含异氰化物的ncAAs可以通过[4 +1]-环加成与四嗪分子选择性反应,从而为位点特异性蛋白质标记和蛋白质降解提供了通用的生物正交处理。重要的是,异氰酸酯和四嗪之间的这些生物正交反应也为激活四嗪猝灭的荧光团提供了独特的机制。将这些含异氰酸酯的ncAA添加到20种常用的天然氨基酸列表中,扩大了我们用于生物正交化学的试剂的范围,因此使新的生物学应用成为可能,从蛋白质标记和成像研究到蛋白质的化学活化。
更新日期:2019-11-14
中文翻译:

在蛋白质标记和激活的遗传密码中添加了含异氰酸酯的氨基酸。
在生物分子中特定位置引入生物正交手柄为研究和操纵蛋白质的结构和功能提供了强大的工具。生物正交化学的最新进展表明,基于四嗪的生物正交环加成因其高反应性,生物选择性和荧光成像的开启特性而成为一种特别有用的方法。尽管其在蛋白质标记和成像中具有广泛的应用,但由于疏水性应变烯烃反应性部分的要求,基于四嗪的生物正交环加成的应用迄今受到限制。绕过此结构要求,我们报告了非标准氨基酸(ncAAs)与小的异氰酸酯(或异腈)基团在细菌和哺乳动物细胞中的蛋白质中的位点特异性结合。我们表明,在生理条件下,在没有催化剂的情况下,这些含异氰化物的ncAAs可以通过[4 +1]-环加成与四嗪分子选择性反应,从而为位点特异性蛋白质标记和蛋白质降解提供了通用的生物正交处理。重要的是,异氰酸酯和四嗪之间的这些生物正交反应也为激活四嗪猝灭的荧光团提供了独特的机制。将这些含异氰酸酯的ncAA添加到20种常用的天然氨基酸列表中,扩大了我们用于生物正交化学的试剂的范围,因此使新的生物学应用成为可能,从蛋白质标记和成像研究到蛋白质的化学活化。































 京公网安备 11010802027423号
京公网安备 11010802027423号