Nature Energy ( IF 49.7 ) Pub Date : 2019-11-04 , DOI: 10.1038/s41560-019-0490-3 Yimin A. Wu , Ian McNulty , Cong Liu , Kah Chun Lau , Qi Liu , Arvydas P. Paulikas , Cheng-Jun Sun , Zhonghou Cai , Jeffrey R. Guest , Yang Ren , Vojislav Stamenkovic , Larry A. Curtiss , Yuzi Liu , Tijana Rajh
|
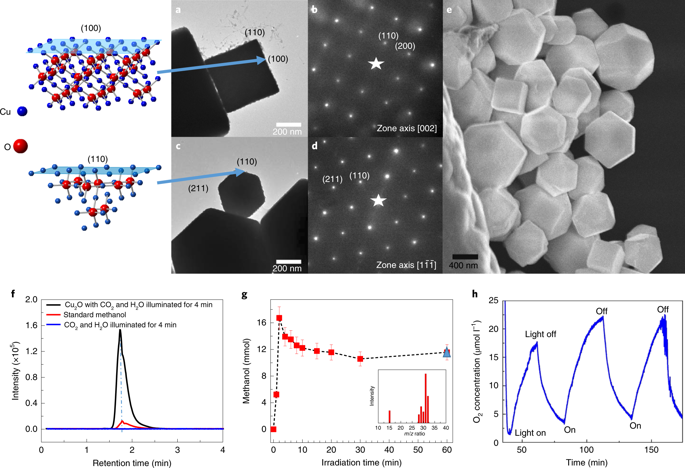
Atomic-level understanding of the active sites and transformation mechanisms under realistic working conditions is a prerequisite for rational design of high-performance photocatalysts. Here, by using correlated scanning fluorescence X-ray microscopy and environmental transmission electron microscopy at atmospheric pressure, in operando, we directly observe that the (110) facet of a single Cu2O photocatalyst particle is photocatalytically active for CO2 reduction to methanol while the (100) facet is inert. The oxidation state of the active sites changes from Cu(i) towards Cu(ii) due to CO2 and H2O co-adsorption and changes back to Cu(i) after CO2 conversion under visible light illumination. The Cu2O photocatalyst oxidizes water as it reduces CO2. Concomitantly, the crystal lattice expands due to CO2 adsorption then reverts after CO2 conversion. The internal quantum yield for unassisted wireless photocatalytic reduction of CO2 to methanol using Cu2O crystals is ~72%.
中文翻译:

单个Cu 2 O颗粒光催化剂上取决于面的活性中心,用于将CO 2还原为甲醇
在实际工作条件下对活性位点和转化机理的原子级了解是合理设计高性能光催化剂的先决条件。在这里,通过在大气压力下使用相关的扫描荧光X射线显微镜和环境透射电子显微镜,在操作中,我们直接观察到单个Cu 2 O光催化剂颗粒的(110)小面具有光催化活性,可将CO 2还原为甲醇,而(100)方面是惰性的。活性位点的氧化态由于CO 2和H 2 O的共吸附而从Cu(i)变为Cu (ii),然后又变回Cu(i)),然后在可见光照射下进行CO 2转化。Cu 2 O光催化剂在还原CO 2时将水氧化。伴随地,由于CO 2吸附,晶格膨胀,然后在CO 2转化后恢复。使用Cu 2 O晶体进行无辅助的无线辅助光催化将CO 2还原为甲醇的内部量子产率为〜72 %。






























 京公网安备 11010802027423号
京公网安备 11010802027423号