当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Breaking the Local Symmetry of LiCoO2 via Atomic Doping for Efficient Oxygen Evolution.
Nano Letters ( IF 9.6 ) Pub Date : 2019-11-07 , DOI: 10.1021/acs.nanolett.9b03523 Zhirong Zhang 1 , Chunxiao Liu 1 , Chen Feng 1 , Pengfei Gao 1 , Yulin Liu 1 , Fangning Ren 1 , Yifeng Zhu 1 , Cong Cao 1 , Wensheng Yan 1 , Rui Si 2 , Shiming Zhou 1 , Jie Zeng 1
Nano Letters ( IF 9.6 ) Pub Date : 2019-11-07 , DOI: 10.1021/acs.nanolett.9b03523 Zhirong Zhang 1 , Chunxiao Liu 1 , Chen Feng 1 , Pengfei Gao 1 , Yulin Liu 1 , Fangning Ren 1 , Yifeng Zhu 1 , Cong Cao 1 , Wensheng Yan 1 , Rui Si 2 , Shiming Zhou 1 , Jie Zeng 1
Affiliation
|
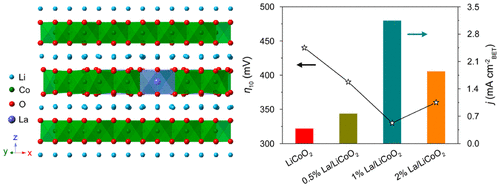
The obstacle for efficient electrochemical water splitting lies in the kinetically sluggish oxygen evolution reaction. Despite the various efforts that have been made to understand and tune the active sites for oxygen evolution reaction, an insight into the configurations of active sites from the electronic perspective is still lacking. Here, we report an atomic doping strategy to break the Oh symmetry of the CoO6 octahedron in LiCoO2. The specific activity of the La-doped LiCoO2 was 3.14 mA cm-2 at the overpotential of 0.35 V, which was 8.3 times higher than that of pristine LiCoO2. The overpotential with a value of 330 mV at 10 mA cm-2 was the lowest among the LiCoO2-based OER electrocatalysts ever reported. Mechanistic studies revealed that the superior activity originated from the asymmetric octahedral coordination of Co, resulting in the enhanced electronic conductivity and Co-O hybridization for the accelerated oxygen evolution kinetics. This work opens a door to enhance the catalytic performance through the manipulation of local symmetry.
中文翻译:

通过原子掺杂打破LiCoO2的局部对称性,以有效释放氧气。
有效的电化学水分解的障碍在于动力学缓慢的氧气析出反应。尽管已经做出了各种努力来理解和调节用于氧释放反应的活性位点,但是仍然缺乏从电子角度对活性位点的构型的了解。在这里,我们报告了一种原子掺杂策略,以打破LiCoO2中CoO6八面体的Oh对称性。La掺杂的LiCoO2在0.35 V的过电势下的比活度为3.14 mA cm-2,是原始LiCoO2的8.3倍。在有报道的基于LiCoO2的OER电催化剂中,在10 mA cm-2处具有330 mV的过电势是最低的。机理研究表明,优异的活性源于Co的不对称八面体配位,导致增强的电子电导率和Co-O杂化以加速氧气释放动力学。这项工作为通过控制局部对称性提高催化性能打开了一扇门。
更新日期:2019-11-07
中文翻译:

通过原子掺杂打破LiCoO2的局部对称性,以有效释放氧气。
有效的电化学水分解的障碍在于动力学缓慢的氧气析出反应。尽管已经做出了各种努力来理解和调节用于氧释放反应的活性位点,但是仍然缺乏从电子角度对活性位点的构型的了解。在这里,我们报告了一种原子掺杂策略,以打破LiCoO2中CoO6八面体的Oh对称性。La掺杂的LiCoO2在0.35 V的过电势下的比活度为3.14 mA cm-2,是原始LiCoO2的8.3倍。在有报道的基于LiCoO2的OER电催化剂中,在10 mA cm-2处具有330 mV的过电势是最低的。机理研究表明,优异的活性源于Co的不对称八面体配位,导致增强的电子电导率和Co-O杂化以加速氧气释放动力学。这项工作为通过控制局部对称性提高催化性能打开了一扇门。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号