当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study of composition and structure of aluminum phosphate binder
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-06-24 , DOI: 10.1002/jccs.201900008 Huixian Wei 1 , Tongjun Wang 1 , Qiang Zhang 2 , Yanwei Jiang 1 , Chenghao Mo 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-06-24 , DOI: 10.1002/jccs.201900008 Huixian Wei 1 , Tongjun Wang 1 , Qiang Zhang 2 , Yanwei Jiang 1 , Chenghao Mo 1
Affiliation
|
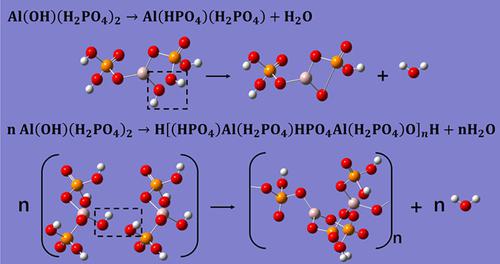
In this article, theoretical analysis and different testing techniques were used to study the reaction pathways and synthesized products of phosphoric acid and aluminum hydroxide at different Al/P molar ratios. The results show that: (a) When the molar ratio of phosphoric acid/aluminum hydroxide is 1:3, the reaction will produce stoichiometric aluminum dihydrogen phosphate (Al(H2PO4)3); (b) when Al(OH)3 is excessive, an intermediate, monohydroxy aluminum dihydrogen phospate (HO‐Al‐(H2PO4)2), will appear, which is unstable and will continue to react according to two reaction pathways, one is intramolecular dehydration to form phosphoric acid hydrogen‐dihydrogen aluminum diphosphate (H2PO4)Al(HPO4); the other is intermolecular dehydration cross‐linking to form a polymeric macromolecular aluminum phosphate H‐((HPO4)(H2PO4)Al‐O‐HPO4‐Al(H2PO4)‐O)‐
nH. The ratio of the two pathways is affected by the excess of Al(OH)3. When the excess of Al(OH)3 continues to increase, the ratio of the second reaction path begins to increase and the viscosity of the product gradually increases. Adhesion experiments show that the aluminum dihydrogen phosphate has the best bonding performance benefiting from its lower viscosity.
中文翻译:

磷酸铝粘结剂的组成和结构研究
本文通过理论分析和不同的测试技术研究了不同Al / P摩尔比的磷酸与氢氧化铝的反应途径和合成产物。结果表明:(a)当磷酸/氢氧化铝的摩尔比为1∶3时,反应将产生化学计量的磷酸二氢铝(Al(H 2 PO 4)3);(b)当Al(OH)3过量时,是一种中间的单羟基磷酸二氢铝(HO‐Al‐(H 2 PO 4)2)会出现,不稳定并会根据两种反应途径继续反应,一种是分子内脱水形成磷酸氢二氢二磷酸铝(H 2 PO 4)Al(HPO 4); 另一个是分子间脱水交联形成聚合的大分子磷酸铝H-(((HPO 4)(H 2 PO 4)Al-O-HPO 4 - Al(H 2 PO 4)-O)-n H. Al(OH)3的过量会影响两个途径的比率。当过量的Al(OH)3 继续增加,第二反应路径的比例开始增加,产物的粘度逐渐增加。粘合实验表明,磷酸二氢铝具有较低的粘度,因此具有最佳的粘合性能。
更新日期:2020-01-23
中文翻译:

磷酸铝粘结剂的组成和结构研究
本文通过理论分析和不同的测试技术研究了不同Al / P摩尔比的磷酸与氢氧化铝的反应途径和合成产物。结果表明:(a)当磷酸/氢氧化铝的摩尔比为1∶3时,反应将产生化学计量的磷酸二氢铝(Al(H 2 PO 4)3);(b)当Al(OH)3过量时,是一种中间的单羟基磷酸二氢铝(HO‐Al‐(H 2 PO 4)2)会出现,不稳定并会根据两种反应途径继续反应,一种是分子内脱水形成磷酸氢二氢二磷酸铝(H 2 PO 4)Al(HPO 4); 另一个是分子间脱水交联形成聚合的大分子磷酸铝H-(((HPO 4)(H 2 PO 4)Al-O-HPO 4 - Al(H 2 PO 4)-O)-n H. Al(OH)3的过量会影响两个途径的比率。当过量的Al(OH)3 继续增加,第二反应路径的比例开始增加,产物的粘度逐渐增加。粘合实验表明,磷酸二氢铝具有较低的粘度,因此具有最佳的粘合性能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号