当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid‐state nuclear magnetic resonance investigation of neurosteroid compounds and magnesium interactions
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-03-28 , DOI: 10.1002/jccs.201800458
Danni Wu,Kathleen Joyce D. Carillo,Shen‐Long Tsai,Jiun‐Jie Shie,Der‐Lii M. Tzou
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-03-28 , DOI: 10.1002/jccs.201800458
Danni Wu,Kathleen Joyce D. Carillo,Shen‐Long Tsai,Jiun‐Jie Shie,Der‐Lii M. Tzou
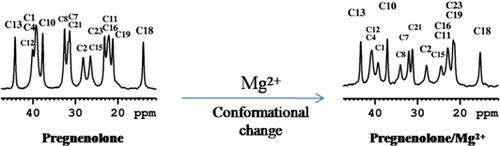
|
The neurosteroid trans‐dehydroandrosterone (DHEA) and its analogs with slightly different modifications in the side chain attached to C17, that is, (3S)‐acetoxypregn‐5‐en‐20‐one (1) and (3S,20R)‐acetoxypregn‐5‐en‐20‐ol (2), have been synthesized to investigate DHEA–cation interactions. In this study, we applied solid‐state 1H/13C cross‐polarization/magic‐angle spinning (CP/MAS) nuclear magnetic resonance (NMR) spectroscopy to a series of DHEA analog/Mg2+ mixtures at different Mg2+ concentrations. The high‐resolution 13C NMR spectra of 1/Mg2+ mixtures exhibit two distinct 13C spectral patterns, one attributable to 1 free from Mg2+, and the other attributable to 1 with bound Mg2+. For 2, the 13C NMR spectra exhibit three distinct spectral patterns; besides that of the free form, the other two can be assigned to Mg2+‐bound forms. Based on the analysis of the chemical shift deviations (CSDs), we conclude that both 1 and 2 might be subject to a cation–π interaction via the C5–C6 double bond, in contrast to that observed previously for DHEA. As demonstrated, DHEA possesses two Mg2+ binding sites, that is, C17–O and C5–C6 double bond, in which the binding affinity of the former is at least three times stronger than that of the latter. The solid‐state 13C NMR investigation allows better understanding of the underlying cation binding effects of neurosteroid molecules in vitro.
中文翻译:

固态核磁共振研究神经甾类化合物和镁的相互作用
神经甾体反式-脱氢雄甾酮(DHEA)及其类似物,其与C17相连的侧链的修饰稍有不同,即(3 S)-乙酰氧基孕烯-5-en-20-一(1)和(3 S,20 R)-acetoxypregn-5-en-20-ol(2)已被合成用于研究DHEA-阳离子的相互作用。在这项研究中,我们将固态1 H / 13 C交叉极化/魔角旋转(CP / MAS)核磁共振(NMR)光谱应用于一系列在不同Mg 2+下的DHEA类似物/ Mg 2+混合物浓度。高分辨率13的C NMR光谱1/ Mg 2+混合物表现出两种不同的13 C光谱图,一种归因于1不含Mg 2+,另一种归因于1具有结合的Mg 2+。为2,该13 C NMR波谱显示出三种不同的光谱模式; 除了自由形式外,其他两个可以分配给Mg 2+结合形式。根据对化学位移偏差(CSD)的分析,我们得出结论,与以前对DHEA观察到的相反,1和2可能都通过C5-C6双键受到阳离子-π相互作用。如图所示,DHEA具有两个Mg2+结合位点,即C17–O和C5–C6双键,其中前者的结合亲和力至少是后者的三倍。固态13 C NMR研究可以更好地了解神经固醇分子在体外的潜在阳离子结合作用。
更新日期:2019-03-28
中文翻译:

固态核磁共振研究神经甾类化合物和镁的相互作用
神经甾体反式-脱氢雄甾酮(DHEA)及其类似物,其与C17相连的侧链的修饰稍有不同,即(3 S)-乙酰氧基孕烯-5-en-20-一(1)和(3 S,20 R)-acetoxypregn-5-en-20-ol(2)已被合成用于研究DHEA-阳离子的相互作用。在这项研究中,我们将固态1 H / 13 C交叉极化/魔角旋转(CP / MAS)核磁共振(NMR)光谱应用于一系列在不同Mg 2+下的DHEA类似物/ Mg 2+混合物浓度。高分辨率13的C NMR光谱1/ Mg 2+混合物表现出两种不同的13 C光谱图,一种归因于1不含Mg 2+,另一种归因于1具有结合的Mg 2+。为2,该13 C NMR波谱显示出三种不同的光谱模式; 除了自由形式外,其他两个可以分配给Mg 2+结合形式。根据对化学位移偏差(CSD)的分析,我们得出结论,与以前对DHEA观察到的相反,1和2可能都通过C5-C6双键受到阳离子-π相互作用。如图所示,DHEA具有两个Mg2+结合位点,即C17–O和C5–C6双键,其中前者的结合亲和力至少是后者的三倍。固态13 C NMR研究可以更好地了解神经固醇分子在体外的潜在阳离子结合作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号