当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel(II) Complexes with Electron-Rich, Sterically Hindered PNP Pincer Ligands Enable Uncommon Modes of Ligand Dearomatization
Organometallics ( IF 2.5 ) Pub Date : 2019-11-01 , DOI: 10.1021/acs.organomet.9b00558 Sébastien Lapointe 1 , Eugene Khaskin 1 , Robert R. Fayzullin 2 , Julia R. Khusnutdinova 1
Organometallics ( IF 2.5 ) Pub Date : 2019-11-01 , DOI: 10.1021/acs.organomet.9b00558 Sébastien Lapointe 1 , Eugene Khaskin 1 , Robert R. Fayzullin 2 , Julia R. Khusnutdinova 1
Affiliation
|
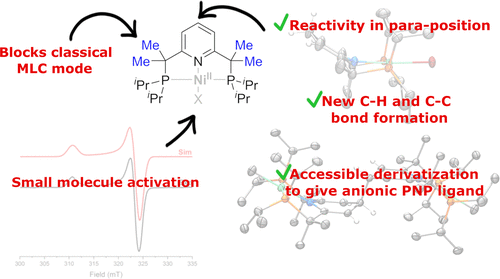
We report the reactivity and characterization of hydride, methyl, and bromo NiII complexes with a new class of electron-rich and sterically hindered PNP pincer ligands, Me4PNPR (R = iPr, tBu), in which a classical metal–ligand cooperative mode of reactivity via CH2 arm deprotonation is blocked by methylation. This enables new, uncommon modes of PNP ligand dearomatization that involve reactivity in the para position of the pyridine ring. In particular, the reduction of [(Me4PNPiPr)NiIIMe]B(ArF)4 with KC8 leads to the formation of a new C–C bond via dimerization of two complexes through the para position. This reactivity stands in sharp contrast to the previously reported bromo or chloro complexes, where stable NiI halogen moieties are formed. Computational analysis showed a greater propensity for ligand-centered radical formation for the presumed intermediate one-electron-reduced species. UV-induced homolysis of the NiII–Me bond in [(Me4PNPiPr)NiIIMe]B(ArF)4 leads to the formation of a Me radical detected by radical traps and NiI intermediates that can be trapped in the presence of halide ions to give previously characterized, stable NiI halogen complexes. In addition, treatment of the bromo complexes [(Me4PNPR)NiIIBr]BPh4 with a powerful hydride source, LiBEt3H, leads to the reduction of the pyridine ring and the formation of NiII complexes with an anionic amide donor reduced pincer ligand, although aromatic NiII hydride complexes could also be obtained with a weaker hydride source. We have observed that steric bulk at the phosphine donors controls the reactivity of the resulting NiIIH complexes. While t-Bu-substituted [(Me4PNPtBu)NiIIH]Y (Y = BPh4, B(ArF)4) does not react with O2, the less sterically hindered iPr-substituted [(Me4PNPiPr)NiIIH]Y reacts instantaneously to give an unstable superoxide adduct that can be observed by EPR.
中文翻译:

镍(II)配合物,带有电子富集的,受位阻的PNP钳形配体,使配体脱芳香化作用的模式变得不常见
我们报告了氢化物,甲基和溴化Ni II配合物与一类新的富含电子和空间受阻的PNP钳形配体Me 4 PNP R(R = i Pr,t Bu)的反应性和表征,其中一种经典金属通过CH 2臂去质子反应的配体协同反应模式被甲基化所阻断。这使得PNP配体脱芳香化的新的,不常见的模式涉及在吡啶环对位的反应性。特别是用KC 8还原[(Me 4 PNP i Pr)Ni II Me] B(Ar F)4通过对位的两个络合物的二聚作用导致新的C–C键的形成。这种反应性与先前报道的溴或氯配合物形成鲜明对比,后者形成了稳定的Ni I卤素部分。计算分析表明,对于假定的中间单电子还原物种,以配体为中心的自由基形成的可能性更大。紫外线诱导的[(Me 4 PNP i Pr)Ni II Me] B(Ar F)4中Ni II -Me键的均相分解导致形成了由自由基陷阱和Ni I检测到的Me自由基可以在存在卤离子的情况下捕获的中间体,以得到先前表征的稳定的Ni I卤素络合物。此外,用强力氢化物源LiBEt 3 H处理溴配合物[(Me 4 PNP R)Ni II Br] BPh 4会导致吡啶环的还原并与阴离子酰胺形成Ni II配合物供体还原的钳位配体,尽管也可以用较弱的氢化物源获得芳族Ni II氢化物络合物。我们已经观察到膦供体上的空间体积控制了所得Ni II H配合物的反应性。虽然t-卜取代的[(ME 4 PNP吨BU)的Ni II H] Y(Y = BPH 4,B(AR ˚F)4)不带O反应2中,较少位阻我镨取代的[(ME 4 PNP我Pr)Ni II H] Y立即反应生成不稳定的超氧化物加合物,可以通过EPR观察到。
更新日期:2019-11-01
中文翻译:

镍(II)配合物,带有电子富集的,受位阻的PNP钳形配体,使配体脱芳香化作用的模式变得不常见
我们报告了氢化物,甲基和溴化Ni II配合物与一类新的富含电子和空间受阻的PNP钳形配体Me 4 PNP R(R = i Pr,t Bu)的反应性和表征,其中一种经典金属通过CH 2臂去质子反应的配体协同反应模式被甲基化所阻断。这使得PNP配体脱芳香化的新的,不常见的模式涉及在吡啶环对位的反应性。特别是用KC 8还原[(Me 4 PNP i Pr)Ni II Me] B(Ar F)4通过对位的两个络合物的二聚作用导致新的C–C键的形成。这种反应性与先前报道的溴或氯配合物形成鲜明对比,后者形成了稳定的Ni I卤素部分。计算分析表明,对于假定的中间单电子还原物种,以配体为中心的自由基形成的可能性更大。紫外线诱导的[(Me 4 PNP i Pr)Ni II Me] B(Ar F)4中Ni II -Me键的均相分解导致形成了由自由基陷阱和Ni I检测到的Me自由基可以在存在卤离子的情况下捕获的中间体,以得到先前表征的稳定的Ni I卤素络合物。此外,用强力氢化物源LiBEt 3 H处理溴配合物[(Me 4 PNP R)Ni II Br] BPh 4会导致吡啶环的还原并与阴离子酰胺形成Ni II配合物供体还原的钳位配体,尽管也可以用较弱的氢化物源获得芳族Ni II氢化物络合物。我们已经观察到膦供体上的空间体积控制了所得Ni II H配合物的反应性。虽然t-卜取代的[(ME 4 PNP吨BU)的Ni II H] Y(Y = BPH 4,B(AR ˚F)4)不带O反应2中,较少位阻我镨取代的[(ME 4 PNP我Pr)Ni II H] Y立即反应生成不稳定的超氧化物加合物,可以通过EPR观察到。

































 京公网安备 11010802027423号
京公网安备 11010802027423号