当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-(3-Aminoazetidin-1-yl)pyrimidin-2-amines as High-Affinity Non-imidazole Histamine H3 Receptor Agonists with in Vivo Central Nervous System Activity.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-11-20 , DOI: 10.1021/acs.jmedchem.9b01462 Gábor Wágner 1 , Tamara A M Mocking 1 , Marta Arimont 1 , Gustavo Provensi , Barbara Rani , Bruna Silva-Marques 2 , Gniewomir Latacz 3 , Daniel Da Costa Pereira 1 , Christina Karatzidou 1 , Henry F Vischer 1 , Maikel Wijtmans 1 , Katarzyna Kieć-Kononowicz 3 , Iwan J P de Esch 1 , Rob Leurs 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-11-20 , DOI: 10.1021/acs.jmedchem.9b01462 Gábor Wágner 1 , Tamara A M Mocking 1 , Marta Arimont 1 , Gustavo Provensi , Barbara Rani , Bruna Silva-Marques 2 , Gniewomir Latacz 3 , Daniel Da Costa Pereira 1 , Christina Karatzidou 1 , Henry F Vischer 1 , Maikel Wijtmans 1 , Katarzyna Kieć-Kononowicz 3 , Iwan J P de Esch 1 , Rob Leurs 1
Affiliation
|
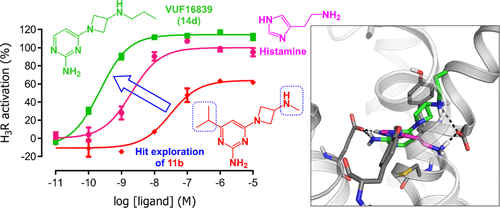
Despite the high diversity of histamine H3 receptor (H3R) antagonist/inverse agonist structures, partial or full H3R agonists have typically been imidazole derivatives. An in-house screening campaign intriguingly afforded the non-imidazole 4-(3-azetidin-1-yl)pyrimidin-2-amine 11b as a partial H3R agonist. Here, the design, synthesis, and structure-activity relationships of 11b analogues are described. This series yields several non-imidazole full agonists with potencies varying with the alkyl substitution pattern on the basic amine following the in vitro evaluation of H3R agonism using a cyclic adenosine monophosphate response element-luciferase reporter gene assay. The key compound VUF16839 (14d) combines nanomolar on-target activity (pKi = 8.5, pEC50 = 9.5) with weak activity on cytochrome P450 enzymes and good metabolic stability. The proposed H3R binding mode of 14d indicates key interactions similar to those attained by histamine. In vivo evaluation of 14d in a social recognition test in mice revealed an amnesic effect at 5 mg/kg intraperitoneally. The excellent in vitro and in vivo pharmacological profiles and the non-imidazole structure of 14d make it a promising tool compound in H3R research.
中文翻译:

4-(3-氨基氮杂环丁烷-1-基)嘧啶-2-胺作为高亲和力非咪唑组胺 H3 受体激动剂,具有体内中枢神经系统活性。
尽管组胺 H3 受体 (H3R) 拮抗剂/反向激动剂结构具有高度多样性,但部分或完全 H3R 激动剂通常是咪唑衍生物。有趣的是,一项内部筛选活动提供了非咪唑 4-(3-氮杂环丁烷-1-基)嘧啶-2-胺 11b 作为部分 H3R 激动剂。这里描述了 11b 类似物的设计、合成和构效关系。该系列产生了几种非咪唑完全激动剂,其效力随碱性胺上的烷基取代模式而变化,并使用环磷酸腺苷响应元件-荧光素酶报告基因测定对 H3R 激动作用进行体外评估。关键化合物 VUF16839 (14d) 兼具纳摩尔级的靶向活性(pKi = 8.5,pEC50 = 9.5)、对细胞色素 P450 酶的弱活性和良好的代谢稳定性。所提出的 14d 的 H3R 结合模式表明与组胺所实现的关键相互作用类似。小鼠社会认知测试中 14 天的体内评估显示,腹腔注射 5 mg/kg 会产生遗忘效应。14d 优异的体外和体内药理学特性以及非咪唑结构使其成为 H3R 研究中很有前途的工具化合物。
更新日期:2019-11-20
中文翻译:

4-(3-氨基氮杂环丁烷-1-基)嘧啶-2-胺作为高亲和力非咪唑组胺 H3 受体激动剂,具有体内中枢神经系统活性。
尽管组胺 H3 受体 (H3R) 拮抗剂/反向激动剂结构具有高度多样性,但部分或完全 H3R 激动剂通常是咪唑衍生物。有趣的是,一项内部筛选活动提供了非咪唑 4-(3-氮杂环丁烷-1-基)嘧啶-2-胺 11b 作为部分 H3R 激动剂。这里描述了 11b 类似物的设计、合成和构效关系。该系列产生了几种非咪唑完全激动剂,其效力随碱性胺上的烷基取代模式而变化,并使用环磷酸腺苷响应元件-荧光素酶报告基因测定对 H3R 激动作用进行体外评估。关键化合物 VUF16839 (14d) 兼具纳摩尔级的靶向活性(pKi = 8.5,pEC50 = 9.5)、对细胞色素 P450 酶的弱活性和良好的代谢稳定性。所提出的 14d 的 H3R 结合模式表明与组胺所实现的关键相互作用类似。小鼠社会认知测试中 14 天的体内评估显示,腹腔注射 5 mg/kg 会产生遗忘效应。14d 优异的体外和体内药理学特性以及非咪唑结构使其成为 H3R 研究中很有前途的工具化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号