Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical Synthesis of Fludarabine and Nelarabine
Synthesis ( IF 2.2 ) Pub Date : 2019-10-30 , DOI: 10.1055/s-0039-1690732 Chunyang Shen , Jiang Liu , Wenliang Ouyang , Haixin Ding , Jiang Bai , Qiang Xiao
Synthesis ( IF 2.2 ) Pub Date : 2019-10-30 , DOI: 10.1055/s-0039-1690732 Chunyang Shen , Jiang Liu , Wenliang Ouyang , Haixin Ding , Jiang Bai , Qiang Xiao
|
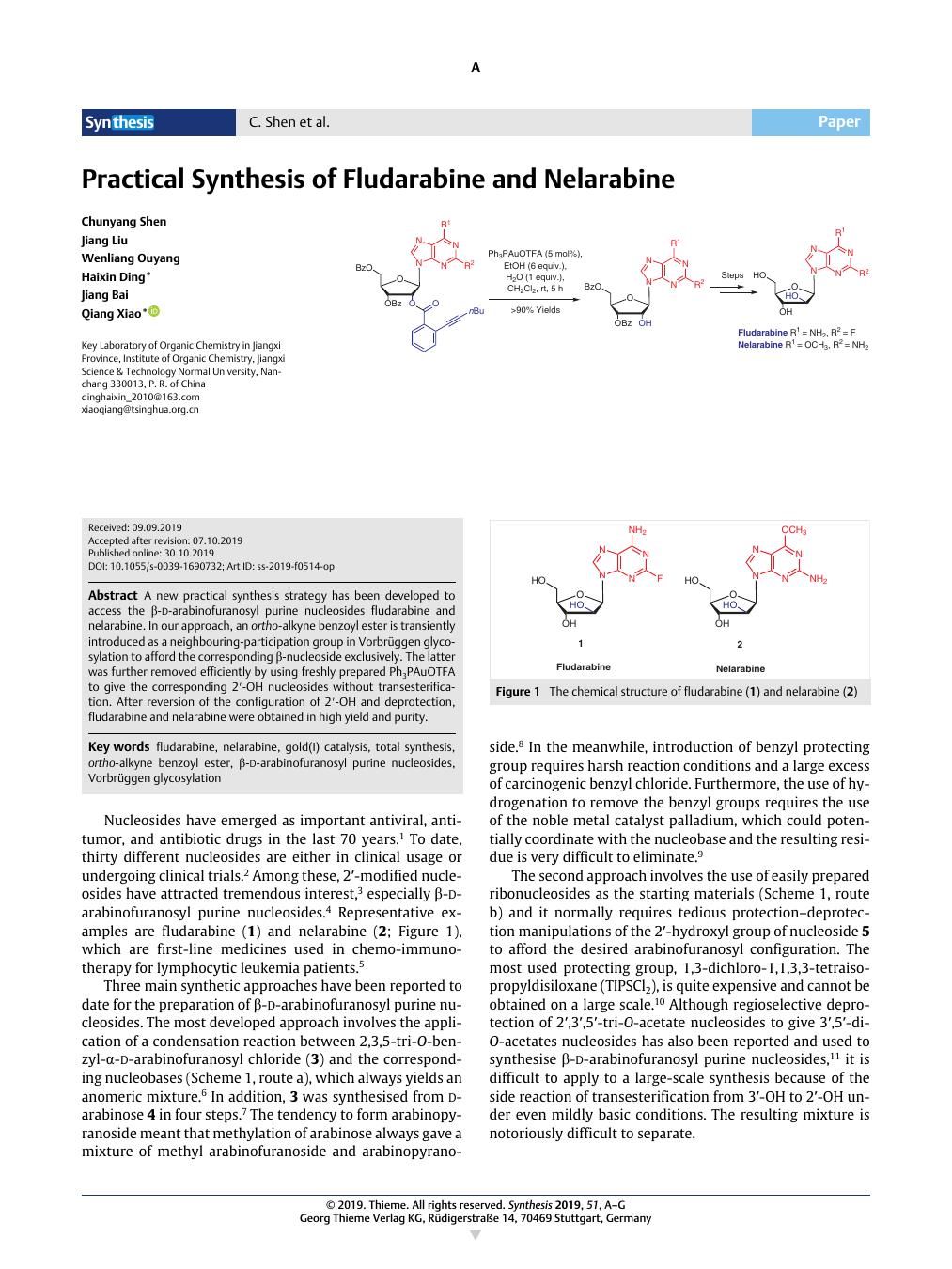
A new practical synthesis strategy has been developed to access the β-d-arabinofuranosyl purine nucleosides fludarabine and nelarabine. In our approach, an ortho-alkyne benzoyl ester is transiently introduced as a neighbouring-participation group in Vorbrüggen glycosylation to afford the corresponding β-nucleoside exclusively. The latter was further removed efficiently by using freshly prepared Ph3PAuOTFA to give the corresponding 2′-OH nucleosides without transesterification. After reversion of the configuration of 2′-OH and deprotection, fludarabine and nelarabine were obtained in high yield and purity.
中文翻译:

氟达拉滨和奈拉拉滨的实用合成
已经开发出一种新的实用合成策略来获得β- d-阿拉伯呋喃糖基嘌呤嘌呤核苷氟达拉滨和奈拉拉滨。在我们的方法中,在Vorbrüggen糖基化中将邻炔基苯甲酸酯作为邻居参与基团暂时引入,以专门提供相应的β-核苷。通过使用新鲜制备的Ph 3 PAuOTFA将后者进一步有效除去,得到相应的2'-OH核苷而不进行酯交换反应。还原2'-OH的构型并脱保护后,可以高收率和纯度获得氟达拉滨和奈拉拉滨。
更新日期:2019-11-01
中文翻译:

氟达拉滨和奈拉拉滨的实用合成
已经开发出一种新的实用合成策略来获得β- d-阿拉伯呋喃糖基嘌呤嘌呤核苷氟达拉滨和奈拉拉滨。在我们的方法中,在Vorbrüggen糖基化中将邻炔基苯甲酸酯作为邻居参与基团暂时引入,以专门提供相应的β-核苷。通过使用新鲜制备的Ph 3 PAuOTFA将后者进一步有效除去,得到相应的2'-OH核苷而不进行酯交换反应。还原2'-OH的构型并脱保护后,可以高收率和纯度获得氟达拉滨和奈拉拉滨。































 京公网安备 11010802027423号
京公网安备 11010802027423号