当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.jconrel.2019.10.028 Pedro P G Guimaraes 1 , Rui Zhang 2 , Roman Spektor 3 , Mingchee Tan 2 , Amanda Chung 2 , Margaret M Billingsley 2 , Rakan El-Mayta 2 , Rachel S Riley 2 , Lili Wang 4 , James M Wilson 4 , Michael J Mitchell 5
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.jconrel.2019.10.028 Pedro P G Guimaraes 1 , Rui Zhang 2 , Roman Spektor 3 , Mingchee Tan 2 , Amanda Chung 2 , Margaret M Billingsley 2 , Rakan El-Mayta 2 , Rachel S Riley 2 , Lili Wang 4 , James M Wilson 4 , Michael J Mitchell 5
Affiliation
|
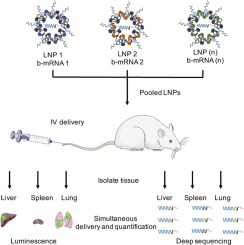
Messenger RNA (mRNA) has recently emerged as a promising class of nucleic acid therapy, with the potential to induce protein production to treat and prevent a range of diseases. However, the widespread use of mRNA as a therapeutic requires safe and effective in vivo delivery technologies. Libraries of ionizable lipid nanoparticles (LNPs) have been designed to encapsulate mRNA, prevent its degradation, and mediate intracellular delivery. However, these LNPs are typically characterized and screened in an in vitro setting, which may not fully replicate the biological barriers that they encounter in vivo. Here, we designed and evaluated a library of engineered LNPs containing barcoded mRNA (b-mRNA) to accelerate the screening of mRNA delivery platforms in vivo. These b-mRNA are similar in structure and function to regular mRNA, and contain barcodes that enable their delivery to be quantified via deep sequencing. Using a mini-library of b-mRNA LNPs formulated via microfluidic mixing, we show that these different formulations can be pooled together, administered intravenously into mice as a single pool, and their delivery to multiple organs (liver, spleen, brain, lung, heart, kidney, pancreas, and muscle) can be quantified simultaneously using deep sequencing. In the context of liver and spleen delivery, LNPs that exhibited high b-mRNA delivery also yielded high luciferase expression, indicating that this platform can identify lead LNP candidates as well as optimal formulation parameters for in vivo mRNA delivery. Interestingly, LNPs with identical formulation parameters that encapsulated different types of nucleic acid barcodes (b-mRNA versus a DNA barcode) altered in vivo delivery, suggesting that the structure of the barcoded nucleic acid affects LNP in vivo delivery. This platform, which enables direct barcoding and subsequent quantification of a functional mRNA, can accelerate the in vivo screening and design of LNPs for mRNA therapeutic applications such as CRISPR-Cas9 gene editing, mRNA vaccination, and other mRNA-based regenerative medicine and protein replacement therapies.
中文翻译:

可电离的脂质纳米粒子封装有条形码的 mRNA,用于加速体内递送筛选。
信使 RNA (mRNA) 最近成为一类有前途的核酸疗法,具有诱导蛋白质产生以治疗和预防一系列疾病的潜力。然而,mRNA 作为治疗剂的广泛使用需要安全有效的体内递送技术。可电离脂质纳米颗粒 (LNP) 文库旨在封装 mRNA、防止其降解并介导细胞内递送。然而,这些 LNP 通常在体外环境中进行表征和筛选,这可能无法完全复制它们在体内遇到的生物屏障。在这里,我们设计并评估了包含条形码 mRNA (b-mRNA) 的工程化 LNP 库,以加速体内 mRNA 递送平台的筛选。这些 b-mRNA 在结构和功能上与常规 mRNA 相似,并且包含条形码,可以通过深度测序对它们的递送进行量化。使用通过微流体混合配制的 b-mRNA LNP 迷你库,我们表明这些不同的制剂可以混合在一起,作为单一池静脉注射到小鼠体内,并将它们递送到多个器官(肝、脾、脑、肺、心脏、肾脏、胰腺和肌肉)可以使用深度测序同时定量。在肝脏和脾脏递送的情况下,表现出高 b-mRNA 递送的 LNP 也产生了高荧光素酶表达,表明该平台可以识别主要 LNP 候选者以及体内 mRNA 递送的最佳制剂参数。有趣的是,封装不同类型核酸条形码(b-mRNA 与 DNA 条形码)的具有相同配方参数的 LNP 改变了体内递送,表明条形码核酸的结构影响 LNP 体内递送。该平台能够对功能性 mRNA 进行直接条形码编码和后续定量,可以加速用于 mRNA 治疗应用(例如 CRISPR-Cas9 基因编辑、mRNA 疫苗接种以及其他基于 mRNA 的再生医学和蛋白质替代)的 LNP 的体内筛选和设计。疗法。
更新日期:2019-10-31
中文翻译:

可电离的脂质纳米粒子封装有条形码的 mRNA,用于加速体内递送筛选。
信使 RNA (mRNA) 最近成为一类有前途的核酸疗法,具有诱导蛋白质产生以治疗和预防一系列疾病的潜力。然而,mRNA 作为治疗剂的广泛使用需要安全有效的体内递送技术。可电离脂质纳米颗粒 (LNP) 文库旨在封装 mRNA、防止其降解并介导细胞内递送。然而,这些 LNP 通常在体外环境中进行表征和筛选,这可能无法完全复制它们在体内遇到的生物屏障。在这里,我们设计并评估了包含条形码 mRNA (b-mRNA) 的工程化 LNP 库,以加速体内 mRNA 递送平台的筛选。这些 b-mRNA 在结构和功能上与常规 mRNA 相似,并且包含条形码,可以通过深度测序对它们的递送进行量化。使用通过微流体混合配制的 b-mRNA LNP 迷你库,我们表明这些不同的制剂可以混合在一起,作为单一池静脉注射到小鼠体内,并将它们递送到多个器官(肝、脾、脑、肺、心脏、肾脏、胰腺和肌肉)可以使用深度测序同时定量。在肝脏和脾脏递送的情况下,表现出高 b-mRNA 递送的 LNP 也产生了高荧光素酶表达,表明该平台可以识别主要 LNP 候选者以及体内 mRNA 递送的最佳制剂参数。有趣的是,封装不同类型核酸条形码(b-mRNA 与 DNA 条形码)的具有相同配方参数的 LNP 改变了体内递送,表明条形码核酸的结构影响 LNP 体内递送。该平台能够对功能性 mRNA 进行直接条形码编码和后续定量,可以加速用于 mRNA 治疗应用(例如 CRISPR-Cas9 基因编辑、mRNA 疫苗接种以及其他基于 mRNA 的再生医学和蛋白质替代)的 LNP 的体内筛选和设计。疗法。































 京公网安备 11010802027423号
京公网安备 11010802027423号