当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient and Sustainable Removal of Magnesium from Brines for Lithium/Magnesium Separation Using Binary Extractants
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-11-12 , DOI: 10.1021/acssuschemeng.9b05436 Zheng Li 1 , Jonas Mercken 1 , Xiaohua Li 1 , Sofía Riaño 1 , Koen Binnemans 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-11-12 , DOI: 10.1021/acssuschemeng.9b05436 Zheng Li 1 , Jonas Mercken 1 , Xiaohua Li 1 , Sofía Riaño 1 , Koen Binnemans 1
Affiliation
|
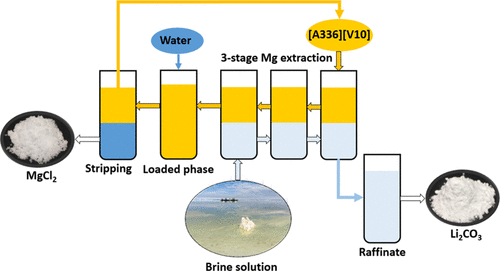
Lithium is becoming increasingly important due to its essential role in lithium-ion batteries. Over 70% of the global lithium resources are found in salt lake brines, but lithium is always accompanied by magnesium. It is a challenge to efficiently separate lithium from magnesium in brines. The state-of-the-art processes for lithium/magnesium separation either consume large quantities of chemicals and generate large amounts of waste or are energy-intensive. In this study, we develop a sustainable solvent extraction process based on binary extractants to efficiently separate lithium and magnesium. A binary extractant composed of Aliquat 336 and Versatic Acid 10, [A336][V10], was prepared and investigated for removal of magnesium from both a (synthetic) concentrated brine (106 g L–1 Mg and 10 g L–1 Li) and an (synthetic) original brine (15 g L–1 Mg, 80 g L–1 Na and 0.2 g L–1 Li). Through batch counter-current experiments and mixer–settler experiments, it was found that [A336][V10] is able to quantitatively remove magnesium from the original brine in three continuous counter-current extraction stages with as little as about 10% coextraction of lithium. The loaded organic phase can be stripped and regenerated by water. The whole process (extraction and stripping) does not consume any acid or base but makes use of the differences in the chloride concentration during extraction and stripping. This process is an environmentally friendly alternative to the state-of-the-art processes and represents a step forward in the sustainable production of Li2CO3 from brines.
中文翻译:

使用二元萃取剂高效,可持续地从卤水中脱除镁,用于锂/镁分离
锂由于其在锂离子电池中的重要作用而变得越来越重要。盐湖盐水中发现了全球70%以上的锂资源,但锂始终伴随着镁。在盐水中有效分离锂和镁是一项挑战。锂/镁分离的最新工艺要么消耗大量化学药品并产生大量废物,要么是能源密集型的。在这项研究中,我们开发了一种基于二元萃取剂的可持续溶剂萃取工艺,以有效分离锂和镁。制备了由Aliquat 336和Versatic Acid 10 [A336] [V10]组成的二元萃取剂,并进行了研究,以从(合成的)浓盐水(106 g L –1 Mg和10 g L –1)中去除镁。Li)和(合成的)原始盐水(15 g L –1 Mg,80 g L –1 Na和0.2 g L –1 Li)。通过分批逆流实验和混合沉降器实验,发现[A336] [V10]能够在三个连续的逆流萃取阶段中以低至约10%的锂共萃取量从原盐水中定量去除镁。 。可以将负载的有机相汽提并用水再生。整个过程(萃取和汽提)不消耗任何酸或碱,而是利用了萃取和汽提过程中氯化物浓度的差异。此工艺是最先进工艺的环保替代方案,代表了锂的可持续生产方面迈出的一步来自盐水的2 CO 3。
更新日期:2019-11-13
中文翻译:

使用二元萃取剂高效,可持续地从卤水中脱除镁,用于锂/镁分离
锂由于其在锂离子电池中的重要作用而变得越来越重要。盐湖盐水中发现了全球70%以上的锂资源,但锂始终伴随着镁。在盐水中有效分离锂和镁是一项挑战。锂/镁分离的最新工艺要么消耗大量化学药品并产生大量废物,要么是能源密集型的。在这项研究中,我们开发了一种基于二元萃取剂的可持续溶剂萃取工艺,以有效分离锂和镁。制备了由Aliquat 336和Versatic Acid 10 [A336] [V10]组成的二元萃取剂,并进行了研究,以从(合成的)浓盐水(106 g L –1 Mg和10 g L –1)中去除镁。Li)和(合成的)原始盐水(15 g L –1 Mg,80 g L –1 Na和0.2 g L –1 Li)。通过分批逆流实验和混合沉降器实验,发现[A336] [V10]能够在三个连续的逆流萃取阶段中以低至约10%的锂共萃取量从原盐水中定量去除镁。 。可以将负载的有机相汽提并用水再生。整个过程(萃取和汽提)不消耗任何酸或碱,而是利用了萃取和汽提过程中氯化物浓度的差异。此工艺是最先进工艺的环保替代方案,代表了锂的可持续生产方面迈出的一步来自盐水的2 CO 3。






























 京公网安备 11010802027423号
京公网安备 11010802027423号