当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of Adsorption Thermodynamics at Electrified Liquid–Solid Interfaces by Electrochemically Modulated Liquid Chromatography
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-11-12 , DOI: 10.1021/acs.jpcc.9b07717 Lisa M. Ponton 1 , David W. Keller 2 , Lorraine M. Siperko , Mark A. Hayes 3 , Marc D. Porter
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-11-12 , DOI: 10.1021/acs.jpcc.9b07717 Lisa M. Ponton 1 , David W. Keller 2 , Lorraine M. Siperko , Mark A. Hayes 3 , Marc D. Porter
Affiliation
|
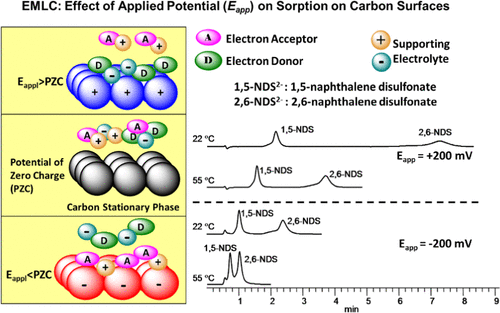
A method to investigate electrosorption thermodynamics has been developed by the examination of the temperature dependence of solute retention by means of electrochemically modulated liquid chromatography (EMLC). This hybrid technique couples electrochemistry and liquid chromatography, which enables the manipulation of solute retention through changes in the potential applied (Eapp) to a conductive stationary phase like glassy carbon, porous graphitic carbon, and boron-doped diamond. An understanding of the thermodynamics of the EMLC separation mechanism would further the interpretations of the rules to predict retention, which may also provide fundamental insights into sorption processes and the structure of the electrical double layer of importance to a number of other technologies (e.g., battery and fuel cells). This paper describes a study in which the dependence of retention for two naphthalene disulfonates (1,5- and 2,6-naphthalene disulfonates) was measured at different fixed values of Eapp [−200 to +200 mV vs Ag/AgCl (sat’d NaCl)] for a glassy carbon chromatographic packing that was held at several different thermally equilibrated column temperatures (22–55 °C). These data were analyzed using the van’t Hoff relationship to determine the enthalpic and entropic contributions to Gibb’s free energy for the transfer of a solute between the mobile and stationary phases. The results for both solutes showed that (1) the retention increased as Eapp moved to more positive values, (2) the retention at each value of Eapp became smaller as the column temperature increased, and (3) the dependence of retention on temperature was stronger as the value of Eapp became more negative. The first and second observations follow expectations for the dependence of the electrostatic interaction strength for anionic solutes on the charge on the electrode surface and for the temperature dependence of the exothermic transfer of a solute from the mobile phase to the stationary phase, respectively. The third observation indicates that retention actually becomes more endothermic as the value of Eapp becomes more positive, which should conceptually cause a decrease rather than an increase in retention. This points to the somewhat surprising importance of entropy to the overall retention mechanism. That is, the increase in retention at increasingly positive values of Eapp reflects the fact that the increase in the entropy for solute transfer has a stronger contribution to the transfer process than the concomitant increase in the endothermicity of transfer. The paper concludes by briefly examining mechanistic origins for the thermodynamic behavior of this system within the context of the electrical double-layer theory and the hydrophobic effect, and possible applications of this intriguing development as a tool for the investigation of electrosorption processes in energy, colloidal, and bioanalytical systems.
中文翻译:

电化学调制液相色谱法研究电-液界面上的吸附热力学
通过利用电化学调制液相色谱法(EMLC)检查溶质保留的温度依赖性,已经开发出一种研究电吸附热力学的方法。这种混合技术结合了电化学和液相色谱,可通过改变所施加的电势来控制溶质的保留(E app)形成导电固定相,如玻璃碳,多孔石墨碳和掺硼金刚石。对EMLC分离机理的热力学的理解将进一步解释预测保留的规则,这也可能提供对吸附过程和双电层结构的基本见解,这对许多其他技术(例如电池)也很重要。和燃料电池)。本文介绍了一项研究,其中在不同的E app固定值下测量了两种萘二磺酸盐(1,5-和2,6-萘二磺酸盐)的保留依赖性。对于玻璃碳色谱填料,[在200至+200 mV相对于Ag / AgCl(饱和的NaCl)]保持在几个不同的热平衡柱温度(22–55°C)。使用范特霍夫(van't Hoff)关系分析了这些数据,以确定了溶质在流动相和固定相之间转移时,焓和熵对吉布自由能的贡献。两种溶质的结果均表明:(1)随着E app移至更大的正值,保留率增加;(2)随着柱温的升高,E app的每个值的保留率均变小;(3)保留率对E app的价值使温度更强变得更加消极。第一和第二个观察分别遵循了对阴离子溶质在电极表面上电荷的静电相互作用强度的依赖性以及溶质从流动相到固定相的放热转移的温度依赖性的期望。第三个观察结果表明,随着E app的值变得更正,保留实际上实际上变得更加吸热,这在概念上应该导致保留的减少而不是增加。这表明熵对整体保留机制的重要性有些令人惊讶。也就是说,在E app的正值越来越高的情况下,保留率提高了反映了这样一个事实,即溶质转移的熵的增加比转移的吸热性的同时增加对转移过程的贡献更大。本文通过在电双层理论和疏水效应的背景下简要检查该系统的热力学行为的机理起源进行了总结,并将这种有趣的开发作为研究能量,胶体中电吸附过程的工具的可能应用以及生物分析系统。
更新日期:2019-11-13
中文翻译:

电化学调制液相色谱法研究电-液界面上的吸附热力学
通过利用电化学调制液相色谱法(EMLC)检查溶质保留的温度依赖性,已经开发出一种研究电吸附热力学的方法。这种混合技术结合了电化学和液相色谱,可通过改变所施加的电势来控制溶质的保留(E app)形成导电固定相,如玻璃碳,多孔石墨碳和掺硼金刚石。对EMLC分离机理的热力学的理解将进一步解释预测保留的规则,这也可能提供对吸附过程和双电层结构的基本见解,这对许多其他技术(例如电池)也很重要。和燃料电池)。本文介绍了一项研究,其中在不同的E app固定值下测量了两种萘二磺酸盐(1,5-和2,6-萘二磺酸盐)的保留依赖性。对于玻璃碳色谱填料,[在200至+200 mV相对于Ag / AgCl(饱和的NaCl)]保持在几个不同的热平衡柱温度(22–55°C)。使用范特霍夫(van't Hoff)关系分析了这些数据,以确定了溶质在流动相和固定相之间转移时,焓和熵对吉布自由能的贡献。两种溶质的结果均表明:(1)随着E app移至更大的正值,保留率增加;(2)随着柱温的升高,E app的每个值的保留率均变小;(3)保留率对E app的价值使温度更强变得更加消极。第一和第二个观察分别遵循了对阴离子溶质在电极表面上电荷的静电相互作用强度的依赖性以及溶质从流动相到固定相的放热转移的温度依赖性的期望。第三个观察结果表明,随着E app的值变得更正,保留实际上实际上变得更加吸热,这在概念上应该导致保留的减少而不是增加。这表明熵对整体保留机制的重要性有些令人惊讶。也就是说,在E app的正值越来越高的情况下,保留率提高了反映了这样一个事实,即溶质转移的熵的增加比转移的吸热性的同时增加对转移过程的贡献更大。本文通过在电双层理论和疏水效应的背景下简要检查该系统的热力学行为的机理起源进行了总结,并将这种有趣的开发作为研究能量,胶体中电吸附过程的工具的可能应用以及生物分析系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号