当前位置:
X-MOL 学术
›
BBA Proteins Proteom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Roles of the N-terminal domain and remote substrate binding subsites in activity of the debranching barley limit dextrinase.
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-10-30 , DOI: 10.1016/j.bbapap.2019.140294 Susan Andersen 1 , Birte Svensson 1 , Marie Sofie Møller 1
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-10-30 , DOI: 10.1016/j.bbapap.2019.140294 Susan Andersen 1 , Birte Svensson 1 , Marie Sofie Møller 1
Affiliation
|
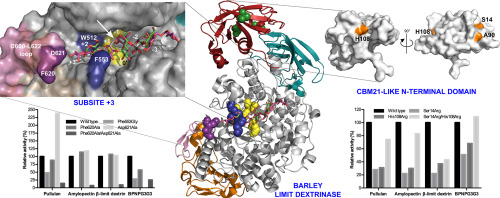
Barley limit dextrinase (HvLD) of glycoside hydrolase family 13 is the sole enzyme hydrolysing α-1,6-glucosidic linkages from starch in the germinating seed. Surprisingly, HvLD shows 150- and 7-fold higher activity towards pullulan and β-limit dextrin, respectively, than amylopectin. This is investigated by mutational analysis of residues in the N-terminal CBM-21-like domain (Ser14Arg, His108Arg, Ser14Arg/His108Arg) and at the outer subsites +2 (Phe553Gly) and +3 (Phe620Ala, Asp621Ala, Phe620Ala/Asp621Ala) of the active site. The Ser14 and His108 mutants mimic natural LD variants from sorghum and rice with elevated enzymatic activity. Although situated about 40 Å from the active site, the single mutants had 15-40% catalytic efficiency compared to wild type for the three polysaccharides and the double mutant retained 27% activity for β-limit dextrin and 64% for pullulan and amylopectin. These three mutants hydrolysed 4,6-O-benzylidene-4-nitrophenyl-63-α-d-maltotriosyl-maltotriose (BPNPG3G3) with 51-109% of wild-type activity. The results highlight that the N-terminal CBM21-like domain plays a role in activity. Phe553 and the highly conserved Trp512 sandwich a substrate main chain glucosyl residue at subsite +2 of the active site, while substrate contacts of Phe620 and Asp621 at subsite +3 are less prominent. Phe553Gly showed 47% and 25% activity on pullulan and BPNPG3G3, respectively having a main role at subsite +2. By contrast at subsite +3, Asp621Ala increased activity on pullulan by 2.4-fold, while Phe620Ala/Asp621Ala retained only 7% activity on pullulan albeit showed 25% activity towards BPNPG3G3. This outcome supports that the outer substrate binding area harbours preference determinants for the branched substrates amylopectin and β-limit dextrin.
中文翻译:

N-末端结构域和远端底物结合亚位点在脱支大麦中的作用限制了糊精酶。
糖苷水解酶家族13的大麦极限糊精酶(HvLD)是从发芽种子中的淀粉中水解α-1,6-糖苷键的唯一酶。令人惊讶的是,HvLD对支链淀粉和β-极限糊精的活性分别比支链淀粉高150倍和7倍。通过突变分析N末端CBM-21-样结构域(Ser14Arg,His108Arg,Ser14Arg / His108Arg)和外部亚位点+2(Phe553Gly)和+3(Phe620Ala,Asp621Ala,Phe620Ala / Asp621Ala)中的残基进行研究活动站点。Ser14和His108突变体模拟高粱和水稻的天然LD变异体,具有较高的酶促活性。尽管距离活动地点约40Å,与三种野生型的野生型相比,单突变体的催化效率为15-40%,而双突变体的β-极限糊精保留27%的活性,支链淀粉和支链淀粉保留64%的活性。这三个突变体水解具有野生型活性的51-109%的4,6-O-亚苄基-4-硝基苯基-63-α-d-麦芽三糖基-麦芽三糖(BPNPG3G3)。结果突出表明,N末端CBM21样结构域在活性中起作用。Phe553和高度保守的Trp512在活性位点的亚位点+2处夹有底物主链葡糖基残基,而在亚位点+3处的Phe620和Asp621的底物接触则不太明显。Phe553Gly对支链淀粉和BPNPG3G3表现出47%和25%的活性,分别在亚位点+2上起主要作用。相反,在亚位点+ 3,Asp621Ala对支链淀粉的活性提高了2.4倍,虽然Phe620Ala / Asp621Ala对支链淀粉仅保留7%的活性,但对BPNPG3G3却显示25%的活性。该结果支持了外底物结合区域具有支链底物支链淀粉和β-极限糊精的偏好决定簇。
更新日期:2019-11-01
中文翻译:

N-末端结构域和远端底物结合亚位点在脱支大麦中的作用限制了糊精酶。
糖苷水解酶家族13的大麦极限糊精酶(HvLD)是从发芽种子中的淀粉中水解α-1,6-糖苷键的唯一酶。令人惊讶的是,HvLD对支链淀粉和β-极限糊精的活性分别比支链淀粉高150倍和7倍。通过突变分析N末端CBM-21-样结构域(Ser14Arg,His108Arg,Ser14Arg / His108Arg)和外部亚位点+2(Phe553Gly)和+3(Phe620Ala,Asp621Ala,Phe620Ala / Asp621Ala)中的残基进行研究活动站点。Ser14和His108突变体模拟高粱和水稻的天然LD变异体,具有较高的酶促活性。尽管距离活动地点约40Å,与三种野生型的野生型相比,单突变体的催化效率为15-40%,而双突变体的β-极限糊精保留27%的活性,支链淀粉和支链淀粉保留64%的活性。这三个突变体水解具有野生型活性的51-109%的4,6-O-亚苄基-4-硝基苯基-63-α-d-麦芽三糖基-麦芽三糖(BPNPG3G3)。结果突出表明,N末端CBM21样结构域在活性中起作用。Phe553和高度保守的Trp512在活性位点的亚位点+2处夹有底物主链葡糖基残基,而在亚位点+3处的Phe620和Asp621的底物接触则不太明显。Phe553Gly对支链淀粉和BPNPG3G3表现出47%和25%的活性,分别在亚位点+2上起主要作用。相反,在亚位点+ 3,Asp621Ala对支链淀粉的活性提高了2.4倍,虽然Phe620Ala / Asp621Ala对支链淀粉仅保留7%的活性,但对BPNPG3G3却显示25%的活性。该结果支持了外底物结合区域具有支链底物支链淀粉和β-极限糊精的偏好决定簇。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号