当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Os-Catalyzed Oxidative Cyclization of 1,5-Dienes.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-11-13 , DOI: 10.1021/acs.joc.9b02174 Aqeel A Hussein 1, 2 , Maximillian J S Phipps 1 , Chris-Kriton Skylaris 1 , Richard C D Brown 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-11-13 , DOI: 10.1021/acs.joc.9b02174 Aqeel A Hussein 1, 2 , Maximillian J S Phipps 1 , Chris-Kriton Skylaris 1 , Richard C D Brown 1
Affiliation
|
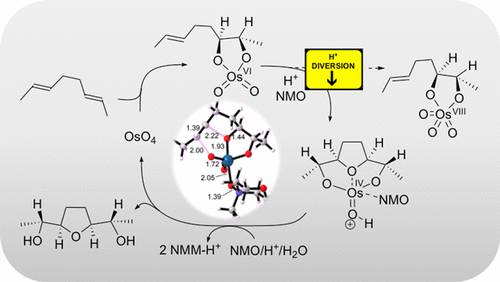
The oxidative cyclization of 1,5-dienes by metal-oxo species is a powerful method for stereocontrolled synthesis of tetrahydrofuran diols (THF-diols), structural motifs present in many bioactive natural products. Oxidative cyclization of (2E,6E)-octa-2,6-diene catalyzed by OsO4/NMO has been studied using density functional theory (DFT) calculations (M06-2X/aug-cc-pVDZ/Hay-Wadt VDZ (n+1) ECP), highlighting the remarkable effect of acid on the fate of the first intermediate, an Os(VI) dioxoglycolate. A strong acid promotes cyclization of the Os(VI) dioxoglycolate, or its NMO complex, through protonation of an oxo ligand to give more electrophilic species. By contrast, in the absence of acid, reoxidation may occur to afford the Os(VIII) trioxoglycolate, which is shown to favor conventional "second cycle" dihydroxylation reactivity rather than cyclization. The results of the calculations are consistent with experimental results for reactions of OsO4/NMO with 1,5-dienes with acid (oxidative cyclization) and without acid (second cycle osmylation/dihydroxylation). Detailed evaluation of potential catalytic cycles supports oxidation of the cyclized Os(IV) THF-diolate intermediate to the corresponding Os(VI) species followed by slow hydrolysis and, finally, regeneration of OsO4.
中文翻译:

Os催化1,5-二烯氧化环化的机理。
金属氧羰基对1,5-二烯的氧化环化是强有力的立体控制合成四氢呋喃二醇(THF-二醇)的方法,四氢呋喃二醇是许多生物活性天然产物中的结构基序。使用密度泛函理论(DFT)计算(M06-2X / aug-cc-pVDZ / Hay-Wadt VDZ(n + 1)ECP),强调了酸对第一中间体Os(VI)二氧乙醇酸酯的显着影响。强酸通过使氧代配体质子化,产生更多的亲电子物质,从而促进Os(VI)二氧代乙醇酸酯或其NMO络合物的环化。相比之下,在不存在酸的情况下,可能会发生重氧化反应,生成Os(VIII)三氧甘醇酸酯,这被证明有利于常规的“第二循环” 二羟基化反应性而不是环化反应。计算结果与OsO4 / NMO与1,5-二烯与酸(氧化环化)和不与酸(第二个循环的osmylation / dihydroxylation)反应的实验结果一致。对潜在催化循环的详细评估支持将环化的Os(IV)THF-二醇酸酯中间体氧化为相应的Os(VI)物种,然后缓慢水解,最后生成OsO4。
更新日期:2019-11-13
中文翻译:

Os催化1,5-二烯氧化环化的机理。
金属氧羰基对1,5-二烯的氧化环化是强有力的立体控制合成四氢呋喃二醇(THF-二醇)的方法,四氢呋喃二醇是许多生物活性天然产物中的结构基序。使用密度泛函理论(DFT)计算(M06-2X / aug-cc-pVDZ / Hay-Wadt VDZ(n + 1)ECP),强调了酸对第一中间体Os(VI)二氧乙醇酸酯的显着影响。强酸通过使氧代配体质子化,产生更多的亲电子物质,从而促进Os(VI)二氧代乙醇酸酯或其NMO络合物的环化。相比之下,在不存在酸的情况下,可能会发生重氧化反应,生成Os(VIII)三氧甘醇酸酯,这被证明有利于常规的“第二循环” 二羟基化反应性而不是环化反应。计算结果与OsO4 / NMO与1,5-二烯与酸(氧化环化)和不与酸(第二个循环的osmylation / dihydroxylation)反应的实验结果一致。对潜在催化循环的详细评估支持将环化的Os(IV)THF-二醇酸酯中间体氧化为相应的Os(VI)物种,然后缓慢水解,最后生成OsO4。































 京公网安备 11010802027423号
京公网安备 11010802027423号