当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Hydrogenolysis of α-C–O Bond in Biomass-Derived 2-Furancarboxylic Acid to 5-Hydroxyvaleric Acid on Supported Pt Catalysts at Near-Ambient Temperature
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-11-13 , DOI: 10.1021/acscatal.9b04074 Qianhui Sun 1, 2 , Shuai Wang 3 , Haichao Liu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-11-13 , DOI: 10.1021/acscatal.9b04074 Qianhui Sun 1, 2 , Shuai Wang 3 , Haichao Liu 1
Affiliation
|
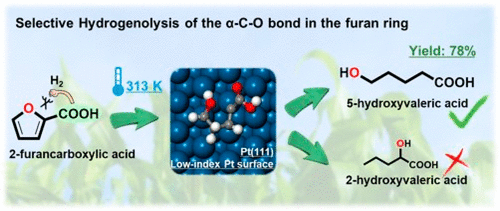
Hydrogenolysis of the α-C–O bond in abundantly available biomass-based furfural and its derivatives provides a viable route for sustainable synthesis of valuable C5 compounds, particularly with two terminal oxygen-containing functional groups. However, efficient cleavage of this bond under mild conditions still remains a crucial challenge, primarily because of the competing cleavage of the δ-C–O bond and hydrogenation of furan ring. Here, we report that supported Pt catalysts were extremely active for the selective α-C–O cleavage in 2-furancarboxylic acid (FCA) hydrogenolysis to synthesize 5-hydroxyvaleric acid (5-HVA), affording a high yield (∼78%) on Pt/SiO2 with a Pt particle size of 4.2 nm at an unprecedentedly low temperature of 313 K. In this reaction, the turnover rate and 5-HVA selectivity sensitively depend on the size of the Pt nanoparticles and the underlying support, as a consequence of their effects on the exposed Pt surfaces. Combined reaction kinetic, infrared spectroscopic, and theoretical assessments reveal that while the exposed high-index Pt surfaces (containing higher fraction of step sites) facilitate the kinetically relevant addition of the first H atom to the unsaturated C atom in furan ring and thus the hydrogenolysis activity, the low-index surfaces (containing higher fraction of terrace sites), together with the electron-withdrawing effect of the carboxylic substituent in FCA, favorably stabilize the dangling C2 atom in the transition states of α-C–O cleavage and lower their activation barriers, leading to the observed high 5-HVA selectivity. Such pivotal roles of the intrinsic properties of metal surfaces and substituents in tuning the reaction pathways will provide a viable strategy for highly selective upgrading of furan derivatives and other biomass-based oxygenates.
中文翻译:

负载型Pt催化剂在近室温下生物质衍生的2-呋喃羧酸中α-C-O键的选择性加氢水解为5-羟基戊酸
大量可用的基于生物质的糠醛及其衍生物中α-C-O键的氢解作用为有价值的C5化合物的可持续合成提供了一条可行的途径,尤其是带有两个末端含氧官能团的化合物。然而,在温和条件下有效裂解该键仍然是一个关键的挑战,主要是因为δ-C-O键的竞争性裂解和呋喃环的氢化。在这里,我们报道了负载的Pt催化剂对于2-呋喃羧酸(FCA)氢解选择性α-C–O裂解以合成5-羟基戊酸(5-HVA)具有极高的活性,收率很高(〜78%)在Pt / SiO 2上在313 K的前所未有的低温下具有4.2 nm的Pt颗粒尺寸。在此反应中,由于其对Pt纳米颗粒和下层载体的影响,其转化率和5-HVA选择性敏感地取决于Pt纳米颗粒和底层载体的尺寸。暴露的铂表面。结合的反应动力学,红外光谱和理论评估表明,尽管暴露的高指数Pt表面(包含较高阶跃部位)有利于将第一个H原子动力学相关地添加到呋喃环中的不饱和C原子上,从而实现氢解活性,低折射率表面(包含更高比例的平台位点)以及FCA中羧基取代基的吸电子作用,有利地将悬垂的C2原子稳定在α-C-O裂解的过渡态并降低其活化势垒,从而导致观察到的高5-HVA选择性。金属表面和取代基的固有性质在调节反应路径中的这种关键作用将为呋喃衍生物和其他基于生物质的含氧化合物的高度选择性提纯提供可行的策略。
更新日期:2019-11-14
中文翻译:

负载型Pt催化剂在近室温下生物质衍生的2-呋喃羧酸中α-C-O键的选择性加氢水解为5-羟基戊酸
大量可用的基于生物质的糠醛及其衍生物中α-C-O键的氢解作用为有价值的C5化合物的可持续合成提供了一条可行的途径,尤其是带有两个末端含氧官能团的化合物。然而,在温和条件下有效裂解该键仍然是一个关键的挑战,主要是因为δ-C-O键的竞争性裂解和呋喃环的氢化。在这里,我们报道了负载的Pt催化剂对于2-呋喃羧酸(FCA)氢解选择性α-C–O裂解以合成5-羟基戊酸(5-HVA)具有极高的活性,收率很高(〜78%)在Pt / SiO 2上在313 K的前所未有的低温下具有4.2 nm的Pt颗粒尺寸。在此反应中,由于其对Pt纳米颗粒和下层载体的影响,其转化率和5-HVA选择性敏感地取决于Pt纳米颗粒和底层载体的尺寸。暴露的铂表面。结合的反应动力学,红外光谱和理论评估表明,尽管暴露的高指数Pt表面(包含较高阶跃部位)有利于将第一个H原子动力学相关地添加到呋喃环中的不饱和C原子上,从而实现氢解活性,低折射率表面(包含更高比例的平台位点)以及FCA中羧基取代基的吸电子作用,有利地将悬垂的C2原子稳定在α-C-O裂解的过渡态并降低其活化势垒,从而导致观察到的高5-HVA选择性。金属表面和取代基的固有性质在调节反应路径中的这种关键作用将为呋喃衍生物和其他基于生物质的含氧化合物的高度选择性提纯提供可行的策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号