当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scaffold-Assisted Ectopic Transplantation of Internal Organs and Patient-Derived Tumors
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2019-11-13 , DOI: 10.1021/acsbiomaterials.9b00978
Ryan Carpenter , Hye Jeong Oh 1 , In-Hye Ham 1 , Daeyoung Kim 2 , Hoon Hur 1 , Jungwoo Lee
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2019-11-13 , DOI: 10.1021/acsbiomaterials.9b00978
Ryan Carpenter , Hye Jeong Oh 1 , In-Hye Ham 1 , Daeyoung Kim 2 , Hoon Hur 1 , Jungwoo Lee
Affiliation
|
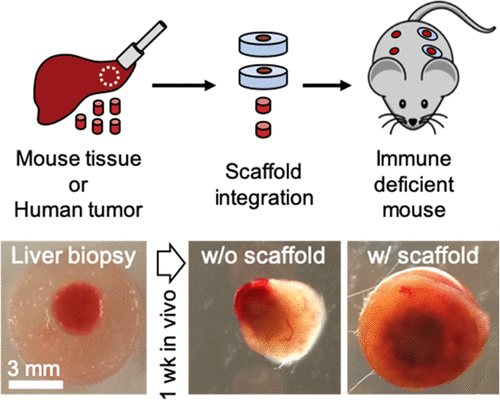
Xenotransplantation of human tissues into immunodeficient mice has emerged as an invaluable preclinical model to study human biology and disease progression and predict clinical response. The most common anatomical site for tissue transplantation is the subcutaneous pocket due to simple surgical procedures and accessibility for gross monitoring and advanced imaging modalities. However, subcutaneously implanted tissues initially experience a sharp change in oxygen and nutrient supply and increased mechanical deformation. During this acute phase of tissue integration to the host vasculature, substantial cell death and tissue fibrosis occur limiting engraftment efficiency. Previously, we demonstrated that the implantation of inverted colloidal crystal hydrogel scaffolds triggers proangiogenic and immunomodulatory functions without characteristic foreign body encapsulation. In this study, we examine the use of this unique host response to improve the ectopic transplantation of tissues to the subcutaneous site. Scaffold-assisted tissues preserved morphological features and blood vessel density compared to native tissues, whereas scaffold-free tissues collapsed and were less vascularized. Notably, the supporting biomaterial scaffold modulated the foreign body response to reduce the localization of Ly6G+ cells within the transplanted tissues. Cotransplantation of patient-derived gastric cancer with a scaffold resulted in a comparable level of engraftment to conventional methods; however, detailed immunohistological characterization revealed significantly better retention of proliferative cells (Ki67+) and human immune cells (CD45+) by the end of the study. We envision that leveraging the immunomodulatory properties of biomaterial interfaces can be an attractive strategy to improve the functional engraftment of xenotransplants and accelerate individualized diagnostics and the development of novel therapeutic strategies.
中文翻译:

支架内脏异位移植和患者源性肿瘤
将人类组织异种移植到免疫缺陷小鼠中已成为一种宝贵的临床前模型,可用于研究人类生物学和疾病进展并预测临床反应。组织移植最常见的解剖部位是皮下袋,这是因为其简单的手术程序以及可进行总体监测和先进的成像方式。但是,皮下植入的组织最初会经历氧气和养分供应的急剧变化,并增加机械变形。在这个组织整合到宿主脉管系统的急性期中,大量的细胞死亡和组织纤维化发生,限制了植入效率。之前,我们证明倒置的胶体晶体水凝胶支架的植入触发了促血管生成和免疫调节功能,而没有特征性的异物包封。在这项研究中,我们研究了使用这种独特的宿主反应来改善异位组织向皮下部位的异位移植。与天然组织相比,支架辅助的组织保留了形态特征和血管密度,而无支架的组织则塌陷且血管化程度降低。值得注意的是,支持性生物材料支架调节异物应答以减少Ly6G的定位 与天然组织相比,支架辅助的组织保留了形态特征和血管密度,而无支架的组织则塌陷且血管化程度降低。值得注意的是,支持性生物材料支架调节异物应答以减少Ly6G的定位 与天然组织相比,支架辅助的组织保留了形态特征和血管密度,而无支架的组织则塌陷且血管化程度降低。值得注意的是,支持性生物材料支架调节异物应答以减少Ly6G的定位+移植组织内的细胞。患者源性胃癌与支架的共移植导致了与传统方法相当的移植水平。然而,详细的免疫组织学表征显示,到研究结束时,增殖细胞(Ki67 +)和人免疫细胞(CD45 +)的滞留性显着提高。我们设想利用生物材料界面的免疫调节特性可以是一种有吸引力的策略,以改善异种移植的功能植入并加速个性化诊断和新型治疗策略的发展。
更新日期:2019-11-13
中文翻译:

支架内脏异位移植和患者源性肿瘤
将人类组织异种移植到免疫缺陷小鼠中已成为一种宝贵的临床前模型,可用于研究人类生物学和疾病进展并预测临床反应。组织移植最常见的解剖部位是皮下袋,这是因为其简单的手术程序以及可进行总体监测和先进的成像方式。但是,皮下植入的组织最初会经历氧气和养分供应的急剧变化,并增加机械变形。在这个组织整合到宿主脉管系统的急性期中,大量的细胞死亡和组织纤维化发生,限制了植入效率。之前,我们证明倒置的胶体晶体水凝胶支架的植入触发了促血管生成和免疫调节功能,而没有特征性的异物包封。在这项研究中,我们研究了使用这种独特的宿主反应来改善异位组织向皮下部位的异位移植。与天然组织相比,支架辅助的组织保留了形态特征和血管密度,而无支架的组织则塌陷且血管化程度降低。值得注意的是,支持性生物材料支架调节异物应答以减少Ly6G的定位 与天然组织相比,支架辅助的组织保留了形态特征和血管密度,而无支架的组织则塌陷且血管化程度降低。值得注意的是,支持性生物材料支架调节异物应答以减少Ly6G的定位 与天然组织相比,支架辅助的组织保留了形态特征和血管密度,而无支架的组织则塌陷且血管化程度降低。值得注意的是,支持性生物材料支架调节异物应答以减少Ly6G的定位+移植组织内的细胞。患者源性胃癌与支架的共移植导致了与传统方法相当的移植水平。然而,详细的免疫组织学表征显示,到研究结束时,增殖细胞(Ki67 +)和人免疫细胞(CD45 +)的滞留性显着提高。我们设想利用生物材料界面的免疫调节特性可以是一种有吸引力的策略,以改善异种移植的功能植入并加速个性化诊断和新型治疗策略的发展。

































 京公网安备 11010802027423号
京公网安备 11010802027423号