当前位置:
X-MOL 学术
›
Cell. Mol. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Loss of Lkb1 impairs Treg function and stability to aggravate graft-versus-host disease after bone marrow transplantation.
Cellular & Molecular Immunology ( IF 21.8 ) Pub Date : 2019-10-29 , DOI: 10.1038/s41423-019-0312-3
Xiuhua Su 1 , Qianqian Wang 1 , Wei Guo 1 , Xiaolei Pei 1 , Qing Niu 1 , Maolan Liu 2 , Yuanyuan Liu 1 , Song Chen 1 , Sizhou Feng 1 , Yi He 1 , Donglin Yang 1 , Rongli Zhang 1 , Qiaoling Ma 1 , Weihua Zhai 1 , Aiming Pang 1 , Jialin Wei 1 , Yong Huang 1 , Yuechen Luo 1 , Mingzhe Han 1 , Xiaoming Feng 1 , Erlie Jiang 1
Cellular & Molecular Immunology ( IF 21.8 ) Pub Date : 2019-10-29 , DOI: 10.1038/s41423-019-0312-3
Xiuhua Su 1 , Qianqian Wang 1 , Wei Guo 1 , Xiaolei Pei 1 , Qing Niu 1 , Maolan Liu 2 , Yuanyuan Liu 1 , Song Chen 1 , Sizhou Feng 1 , Yi He 1 , Donglin Yang 1 , Rongli Zhang 1 , Qiaoling Ma 1 , Weihua Zhai 1 , Aiming Pang 1 , Jialin Wei 1 , Yong Huang 1 , Yuechen Luo 1 , Mingzhe Han 1 , Xiaoming Feng 1 , Erlie Jiang 1
Affiliation
|
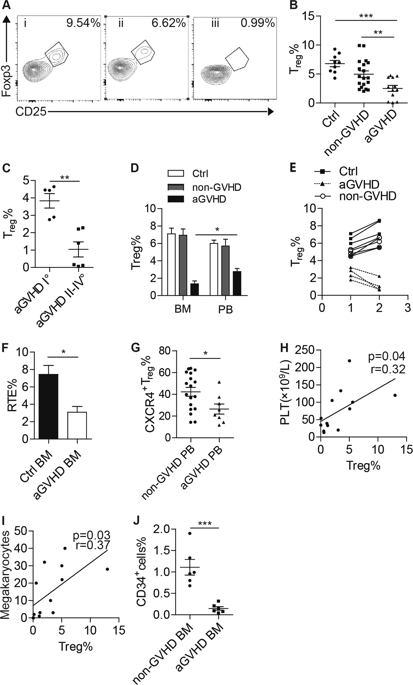
Accumulating evidence suggests that a reduction in the number of Foxp3+ regulatory T cells (Tregs) contributes to the pathogenesis of acute graft-versus-host disease (aGVHD), which is a major adverse complication that can occur after allogeneic hematopoietic stem cell transplantation (allo-HSCT). However, the precise features and mechanism underlying the defects in Tregs remain largely unknown. In this study, we demonstrated that Tregs were more dramatically decreased in bone marrow compared with those in peripheral blood from aGVHD patients and that bone marrow Treg defects were negatively associated with hematopoietic reconstitution. Tregs from aGVHD patients exhibited multiple defects, including the instability of Foxp3 expression, especially in response to IL-12, impaired suppressor function, decreased migratory capacity, and increased apoptosis. Transcriptional profiling revealed the downregulation of Lkb1, a previously identified critical regulator of murine Treg identity and metabolism, and murine Lkb1-regulated genes in Tregs from aGVHD patients. Foxp3 expression in human Tregs could be decreased and increased by the knockdown and overexpression of the Lkb1 gene, respectively. Furthermore, a loss-of-function assay in an aGVHD murine model confirmed that Lkb1 deficiency could impair Tregs and aggravate disease severity. These findings reveal that Lkb1 downregulation contributes to multiple defects in Tregs in human aGVHD and highlight the Lkb1-related pathways that could serve as therapeutic targets that may potentially be manipulated to mitigate aGVHD.
中文翻译:

Lkb1的丧失会损害Treg的功能和稳定性,加重骨髓移植后的移植物抗宿主病。
越来越多的证据表明,Foxp3 +调节性T细胞(Tregs)数量的减少有助于急性移植物抗宿主病(aGVHD)的发病,这是同种异体造血干细胞移植后可能发生的主要不良并发症(allo -HSCT)。但是,Tregs缺陷背后的确切特征和机制仍然未知。在这项研究中,我们证明,与aGVHD患者的外周血相比,骨髓中的Treg降低更为显着,并且骨髓Treg缺陷与造血重建负相关。来自aGVHD患者的Treg表现出多种缺陷,包括Foxp3表达的不稳定性,尤其是对IL-12的响应,抑制功能受损,迁移能力降低,并增加细胞凋亡。转录谱分析揭示了Lkb1的下调,这是先前鉴定的鼠Treg身份和代谢的关键调节剂,以及来自aGVHD患者的Treg中鼠Lkb1调节的基因。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。先前确定的鼠Treg身份和代谢的关键调节剂,以及来自aGVHD患者的Treg中鼠Lkb1调节的基因。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。先前确定的鼠Treg身份和代谢的关键调节剂,以及来自aGVHD患者的Treg中鼠Lkb1调节的基因。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。
更新日期:2019-10-29
中文翻译:

Lkb1的丧失会损害Treg的功能和稳定性,加重骨髓移植后的移植物抗宿主病。
越来越多的证据表明,Foxp3 +调节性T细胞(Tregs)数量的减少有助于急性移植物抗宿主病(aGVHD)的发病,这是同种异体造血干细胞移植后可能发生的主要不良并发症(allo -HSCT)。但是,Tregs缺陷背后的确切特征和机制仍然未知。在这项研究中,我们证明,与aGVHD患者的外周血相比,骨髓中的Treg降低更为显着,并且骨髓Treg缺陷与造血重建负相关。来自aGVHD患者的Treg表现出多种缺陷,包括Foxp3表达的不稳定性,尤其是对IL-12的响应,抑制功能受损,迁移能力降低,并增加细胞凋亡。转录谱分析揭示了Lkb1的下调,这是先前鉴定的鼠Treg身份和代谢的关键调节剂,以及来自aGVHD患者的Treg中鼠Lkb1调节的基因。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。先前确定的鼠Treg身份和代谢的关键调节剂,以及来自aGVHD患者的Treg中鼠Lkb1调节的基因。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。先前确定的鼠Treg身份和代谢的关键调节剂,以及来自aGVHD患者的Treg中鼠Lkb1调节的基因。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。Lkb1基因的敲低和过表达可以分别降低和提高人类Tregs中Foxp3的表达。此外,在aGVHD小鼠模型中进行的功能丧失测定证实Lkb1缺乏会损害Tregs并加重疾病的严重程度。这些发现表明,Lkb1的下调会导致人类aGVHD中Treg的多个缺陷,并突显了Lkb1相关的途径,可作为治疗靶标,可能被操纵以减轻aGVHD。

































 京公网安备 11010802027423号
京公网安备 11010802027423号