当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CSF1R- and SHP2-Inhibitor-Loaded Nanoparticles Enhance Cytotoxic Activity and Phagocytosis in Tumor-Associated Macrophages.
Advanced Materials ( IF 27.4 ) Pub Date : 2019-10-29 , DOI: 10.1002/adma.201904364 Anujan Ramesh 1, 2 , Sahana Kumar 1 , Dipika Nandi 3 , Ashish Kulkarni 1, 2, 3, 4
Advanced Materials ( IF 27.4 ) Pub Date : 2019-10-29 , DOI: 10.1002/adma.201904364 Anujan Ramesh 1, 2 , Sahana Kumar 1 , Dipika Nandi 3 , Ashish Kulkarni 1, 2, 3, 4
Affiliation
|
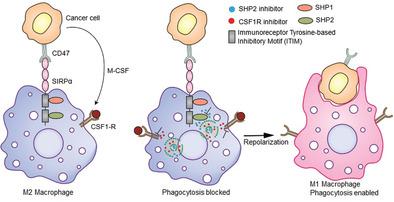
Immune modulation of macrophages has emerged as an attractive approach for anti-cancer therapy. However, there are two main challenges in successfully utilizing macrophages for immunotherapy. First, macrophage colony stimulating factor (MCSF) secreted by cancer cells binds to colony stimulating factor 1 receptor (CSF1-R) on macrophages and in turn activates the downstream signaling pathway responsible for polarization of tumor-associated macrophages (TAMs) to immunosuppressive M2 phenotype. Second, ligation of signal regulatory protein α (SIRPα) expressed on myeloid cells to CD47, a transmembrane protein overexpressed on cancer cells, activates the Src homology region 2 (SH2) domain -phosphatases SHP-1 and SHP-2 in macrophages. This results in activation of "eat-me-not" signaling pathway and inhibition of phagocytosis. Here, it is reported that self-assembled dual-inhibitor-loaded nanoparticles (DNTs) target M2 macrophages and simultaneously inhibit CSF1R and SHP2 pathways. This results in efficient repolarization of M2 macrophages to an active M1 phenotype, and superior phagocytic capabilities as compared to individual drug treatments. Furthermore, suboptimal dose administration of DNTs in highly aggressive breast cancer and melanoma mouse models show enhanced anti-tumor efficacy without any toxicity. These studies demonstrate that the concurrent inhibition of CSF1-R and SHP2 signaling pathways for macrophage activation and phagocytosis enhancement could be an effective strategy for macrophage-based immunotherapy.
中文翻译:

载有CSF1R和SHP2抑制剂的纳米颗粒可增强肿瘤相关巨噬细胞的细胞毒活性和吞噬作用。
巨噬细胞的免疫调节已成为抗癌治疗的一种有吸引力的方法。然而,成功利用巨噬细胞进行免疫治疗存在两个主要挑战。首先,癌细胞分泌的巨噬细胞集落刺激因子(MCSF)与巨噬细胞上的集落刺激因子1受体(CSF1-R)结合,进而激活下游信号通路,该通路负责将肿瘤相关巨噬细胞(TAM)极化为免疫抑制性M2表型。其次,将在髓样细胞上表达的信号调节蛋白α(SIRPα)与CD47(一种在癌细胞上过表达的跨膜蛋白)连接,激活Src同源区域2(SH2)域-磷酸化巨噬细胞中的SHP-1和SHP-2。这导致“非我吃”信号传导途径的激活和吞噬作用的抑制。这里,据报道,自组装的双重抑制剂负载纳米粒子(DNTs)靶向M2巨噬细胞,同时抑制CSF1R和SHP2途径。与单个药物治疗相比,这导致M2巨噬细胞有效地复极化为活跃的M1表型,并具有更高的吞噬能力。此外,在高度侵袭性乳腺癌和黑色素瘤小鼠模型中对DNT的次适量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。与单个药物治疗相比,这导致M2巨噬细胞有效地复极化为活跃的M1表型,并具有更高的吞噬能力。此外,在高度侵袭性乳腺癌和黑色素瘤小鼠模型中对DNT的次适量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。与单个药物治疗相比,这导致M2巨噬细胞有效地复极化为活跃的M1表型,并具有更高的吞噬能力。此外,在高度侵袭性乳腺癌和黑色素瘤小鼠模型中对DNT的次适量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。在高度侵袭性乳腺癌和黑色素瘤小鼠模型中DNT的次适量剂量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。在高度侵袭性乳腺癌和黑色素瘤小鼠模型中DNT的次适量剂量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。
更新日期:2019-12-18
中文翻译:

载有CSF1R和SHP2抑制剂的纳米颗粒可增强肿瘤相关巨噬细胞的细胞毒活性和吞噬作用。
巨噬细胞的免疫调节已成为抗癌治疗的一种有吸引力的方法。然而,成功利用巨噬细胞进行免疫治疗存在两个主要挑战。首先,癌细胞分泌的巨噬细胞集落刺激因子(MCSF)与巨噬细胞上的集落刺激因子1受体(CSF1-R)结合,进而激活下游信号通路,该通路负责将肿瘤相关巨噬细胞(TAM)极化为免疫抑制性M2表型。其次,将在髓样细胞上表达的信号调节蛋白α(SIRPα)与CD47(一种在癌细胞上过表达的跨膜蛋白)连接,激活Src同源区域2(SH2)域-磷酸化巨噬细胞中的SHP-1和SHP-2。这导致“非我吃”信号传导途径的激活和吞噬作用的抑制。这里,据报道,自组装的双重抑制剂负载纳米粒子(DNTs)靶向M2巨噬细胞,同时抑制CSF1R和SHP2途径。与单个药物治疗相比,这导致M2巨噬细胞有效地复极化为活跃的M1表型,并具有更高的吞噬能力。此外,在高度侵袭性乳腺癌和黑色素瘤小鼠模型中对DNT的次适量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。与单个药物治疗相比,这导致M2巨噬细胞有效地复极化为活跃的M1表型,并具有更高的吞噬能力。此外,在高度侵袭性乳腺癌和黑色素瘤小鼠模型中对DNT的次适量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。与单个药物治疗相比,这导致M2巨噬细胞有效地复极化为活跃的M1表型,并具有更高的吞噬能力。此外,在高度侵袭性乳腺癌和黑色素瘤小鼠模型中对DNT的次适量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。在高度侵袭性乳腺癌和黑色素瘤小鼠模型中DNT的次适量剂量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。在高度侵袭性乳腺癌和黑色素瘤小鼠模型中DNT的次适量剂量给药显示出增强的抗肿瘤功效,而没有任何毒性。这些研究表明,同时抑制CSF1-R和SHP2信号通路对巨噬细胞的激活和吞噬作用的增强可能是基于巨噬细胞的免疫治疗的有效策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号