当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Uncommon Behavior of Tetra-alkyl-phosphonium 2-Cyano-pyrrolide Ionic Liquids + Glycerol and Triethanolamine Systems
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-10-28 , DOI: 10.1021/acs.jced.9b00769 Oscar Nordness 1 , Oscar Morales-Collazo 1 , Joan F. Brennecke 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-10-28 , DOI: 10.1021/acs.jced.9b00769 Oscar Nordness 1 , Oscar Morales-Collazo 1 , Joan F. Brennecke 1
Affiliation
|
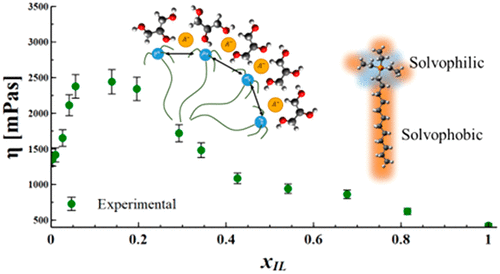
We use multiple characterization methods, including viscosity, ionic conductivity, density, and surface tension measurements, to investigate the unusual transport behavior of multiple tetra-alkyl-phosphonium-2-cyano-pyrrolide ([Pnnnm][2CNPyr]) ILs in glycerol and triethanolamine solvents. Specifically, the viscosity goes through a maximum for all of the IL/glycerol and IL/triethanolamine mixtures, which cannot be explained by traditional mixing rules. Through conductivity and surface tension characterization we find substantial evidence of microstructure formation driven by the amphiphilic structure of the tetra-alkyl-phosphonium ([Pnnnm]+) cation accounting for the uncommon viscosity behavior. While micelle formation is well-known for IL+ water systems, the observation of these microstructures in nonaqueous systems is a significant discovery. The slope of the ionic conductivity as a function of IL concentration changes substantially at the critical aggregate concentration cac, at which point the ILs undergo solvophobic self-assembly; this phenomenon is observed in all IL/solvent mixtures investigated. The cac is observed at more dilute IL concentrations for ILs containing longer alkyl chains. Furthermore, we observe lower cac values in glycerol compared to that of triethanolamine, owing to its higher dielectric constant and larger cohesion energy, as measured by the Gordon parameter.
中文翻译:

四烷基-2-氰基吡咯化物离子液体+甘油和三乙醇胺系统的不寻常行为
我们使用多种表征方法,包括粘度,离子电导率,密度和表面张力的测量,调查多个四烷基鏻-2-氰基吡咯的不寻常的运输行为([P nnnm ] [2CNPyr])在甘油中离子液体和三乙醇胺溶剂。特别地,对于所有的IL /甘油和IL /三乙醇胺混合物,粘度都达到最大值,这不能用传统的混合规则来解释。通过电导率和表面张力表征,我们找到了由四烷基-([P nnnm ] +)阳离子解释了不常见的粘度行为。尽管对于IL +水系统来说,胶束的形成是众所周知的,但在非水系统中观察到这些微结构却是一个重大发现。离子电导率随IL浓度变化的斜率在临界聚集体浓度cac时发生很大变化,此时IL经历了疏溶剂性自组装。在研究的所有IL /溶剂混合物中均观察到此现象。对于包含更长烷基链的IL,可以在更稀的IL浓度下观察到cac。此外,与甘油三乙醇胺相比,我们观察到甘油的cac值较低,这是由于其较高的介电常数和较大的内聚能(通过Gordon参数测得)。
更新日期:2019-10-28
中文翻译:

四烷基-2-氰基吡咯化物离子液体+甘油和三乙醇胺系统的不寻常行为
我们使用多种表征方法,包括粘度,离子电导率,密度和表面张力的测量,调查多个四烷基鏻-2-氰基吡咯的不寻常的运输行为([P nnnm ] [2CNPyr])在甘油中离子液体和三乙醇胺溶剂。特别地,对于所有的IL /甘油和IL /三乙醇胺混合物,粘度都达到最大值,这不能用传统的混合规则来解释。通过电导率和表面张力表征,我们找到了由四烷基-([P nnnm ] +)阳离子解释了不常见的粘度行为。尽管对于IL +水系统来说,胶束的形成是众所周知的,但在非水系统中观察到这些微结构却是一个重大发现。离子电导率随IL浓度变化的斜率在临界聚集体浓度cac时发生很大变化,此时IL经历了疏溶剂性自组装。在研究的所有IL /溶剂混合物中均观察到此现象。对于包含更长烷基链的IL,可以在更稀的IL浓度下观察到cac。此外,与甘油三乙醇胺相比,我们观察到甘油的cac值较低,这是由于其较高的介电常数和较大的内聚能(通过Gordon参数测得)。































 京公网安备 11010802027423号
京公网安备 11010802027423号