当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Olefin Ring‐Opening Cross Metathesis Catalyzed by Molybdenum Imido Alkylidene N‐Heterocyclic Carbene Complexes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-11-07 , DOI: 10.1002/adsc.201900979
Mohasin Momin 1 , Gergely M. Nagy 1 , Michael R. Buchmeiser 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-11-07 , DOI: 10.1002/adsc.201900979
Mohasin Momin 1 , Gergely M. Nagy 1 , Michael R. Buchmeiser 1, 2
Affiliation
|
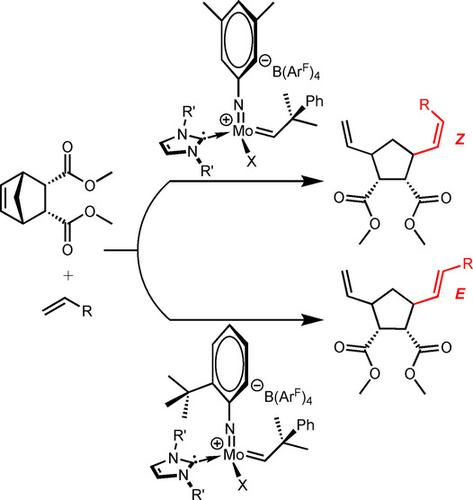
The influence of the structure of cationic molybdenum imido alkylidene N‐heterocyclic carbene (NHC) catalysts, i. e. of [Mo(N‐2‐tert‐butyl‐C6H4) (CHCMe2Ph)(NHC)X+ B(ArF)4−] (NHC=1,3‐di(2‐Pr)imidazol‐2‐ylidene (iPr), 1,3‐dimesitylimidazol‐2‐ylidene (IMes); X=pyrrolide, OCH(CF3)2, B(ArF)4−=tetrakis(3,5‐bis(trifluoromethyl)phenyl)borate) and of [Mo(N‐3,5‐Me2‐C6H3)(CHCMe2Ph)(NHC)(CH3CN)X+ B(ArF)4−] (NHC=1,3‐dimesitylimidazol‐2‐ylidene, 1,3‐dimesitylimidazolin‐2‐ylidene (IMesH2); X=CF3SO3, OCPh(CF3)2) on E/Z‐selectivity in the ring‐opening cross‐metathesis (ROCM) of endo, endo‐2,3‐dicarbomethoxynorborn‐5‐ene (endo, endo‐DCMNBE), exo, exo‐2,3‐dicarbomethoxynorborn‐5‐ene (exo, exo‐DCMNBE), endo, exo‐2,3‐dicarbomethoxynorborn‐5‐ene ((+) DCMNBE) and 2,3‐exo,exo‐bis(acetoxymethyl)‐7‐oxabicyclo[2.2.l]hept‐5‐ene (7‐oxa‐NBE) with 1‐pentene, styrene, allyltrimethylsilane, allyl benzyl ether, allyl phenyl ether and allyl ethyl ether has been studied. With the exception of the ROCM reaction of endo, endo‐DCMNBE with styrene, all other ROCM reactions of endo, endo‐DCMNBE proceeded under thermodynamic control without any post‐metathesis isomerization reactions with full retention of the configuration of the newly formed 1,2‐disubstituted double bond as confirmed by kinetic studies. Similar accounts for selected homometathesis reactions. Catalyst structure‐selectivity correlations based on the buried volume, Vbur, of the N‐imido ligand are presented.
中文翻译:

钼亚胺基亚烷基N-杂环碳烯配合物催化的立体选择性烯烃开环交叉易位
阳离子钼亚胺基亚烷基N杂环卡宾(NHC)催化剂的结构影响,i。e。的[沫(N-2-叔丁基-C 6 H ^ 4)(CHCMe 2 PH)(NHC)X + B(氩˚F)4 - ](NHC = 1,3-二(2-PR)咪唑咪唑-2-亚基(我PR),1,3- dimesitylimidazol -2-亚基(IMES); X =吡咯,OCH(CF 3)2,B(AR ˚F)4 - =四(3,5-双(三氟甲基) )和([Mo(N-3,5-Me 2 -C 6 H 3)(CHCMe 2Ph)(NHC)(CH 3 CN)X + B(Ar F)4 − ](NHC = 1,3-二甲酰亚胺基-2-亚基,1,3-二咪唑啉-2-基(IMesH 2); X = CF 3 SO 3,OCPh(CF 3)2)对内,内-2,3-二苯甲氧基降冰片烯5-内烯(内,内-DCMNBE)的开环交叉复分解(ROCM)的E / Z选择性,exo,exo -2,3-二苯甲氧基降冰片烯5-烯(exo,exo- DCMNBE),内酯,exo -2,3-二苯甲氧基降冰片烯((+)DCMNBE)和2,3 -exo,exo-bis研究了(乙酰氧基甲基)-7-氧杂双环[2.2.l]庚-5-烯(7-氧杂-NBE)与1-戊烯,苯乙烯,烯丙基三甲基硅烷,烯丙基苄基醚,烯丙基苯基醚和烯丙基乙基醚的关系。用的反应ROCM的例外内,内切与苯乙烯-DCMNBE,所有其他ROCM反应内,内切热力学控制下进行时没有与新形成的1,2-结构的完全保留任何后易位异构化反应-DCMNBE动力学研究证实了双取代的双键。对于选择的同质置换反应也有类似的解释。基于掩埋体积催化剂结构选择性的相关性,V BUR所述的,Ñ配体-imido被呈现。
更新日期:2019-11-07
中文翻译:

钼亚胺基亚烷基N-杂环碳烯配合物催化的立体选择性烯烃开环交叉易位
阳离子钼亚胺基亚烷基N杂环卡宾(NHC)催化剂的结构影响,i。e。的[沫(N-2-叔丁基-C 6 H ^ 4)(CHCMe 2 PH)(NHC)X + B(氩˚F)4 - ](NHC = 1,3-二(2-PR)咪唑咪唑-2-亚基(我PR),1,3- dimesitylimidazol -2-亚基(IMES); X =吡咯,OCH(CF 3)2,B(AR ˚F)4 - =四(3,5-双(三氟甲基) )和([Mo(N-3,5-Me 2 -C 6 H 3)(CHCMe 2Ph)(NHC)(CH 3 CN)X + B(Ar F)4 − ](NHC = 1,3-二甲酰亚胺基-2-亚基,1,3-二咪唑啉-2-基(IMesH 2); X = CF 3 SO 3,OCPh(CF 3)2)对内,内-2,3-二苯甲氧基降冰片烯5-内烯(内,内-DCMNBE)的开环交叉复分解(ROCM)的E / Z选择性,exo,exo -2,3-二苯甲氧基降冰片烯5-烯(exo,exo- DCMNBE),内酯,exo -2,3-二苯甲氧基降冰片烯((+)DCMNBE)和2,3 -exo,exo-bis研究了(乙酰氧基甲基)-7-氧杂双环[2.2.l]庚-5-烯(7-氧杂-NBE)与1-戊烯,苯乙烯,烯丙基三甲基硅烷,烯丙基苄基醚,烯丙基苯基醚和烯丙基乙基醚的关系。用的反应ROCM的例外内,内切与苯乙烯-DCMNBE,所有其他ROCM反应内,内切热力学控制下进行时没有与新形成的1,2-结构的完全保留任何后易位异构化反应-DCMNBE动力学研究证实了双取代的双键。对于选择的同质置换反应也有类似的解释。基于掩埋体积催化剂结构选择性的相关性,V BUR所述的,Ñ配体-imido被呈现。

































 京公网安备 11010802027423号
京公网安备 11010802027423号