Cellular & Molecular Immunology ( IF 21.8 ) Pub Date : 2019-10-28 , DOI: 10.1038/s41423-019-0316-z Kelsey Voss 1 , Camille Lake 1 , Christopher R Luthers 1 , Nathaniel M Lott 2 , Batsukh Dorjbal 1 , Swadhinya Arjunaraja 1 , Bradly M Bauman 1 , Anthony R Soltis 2 , Gauthaman Sukumar 2 , Clifton L Dalgard 2, 3 , Andrew L Snow 1
|
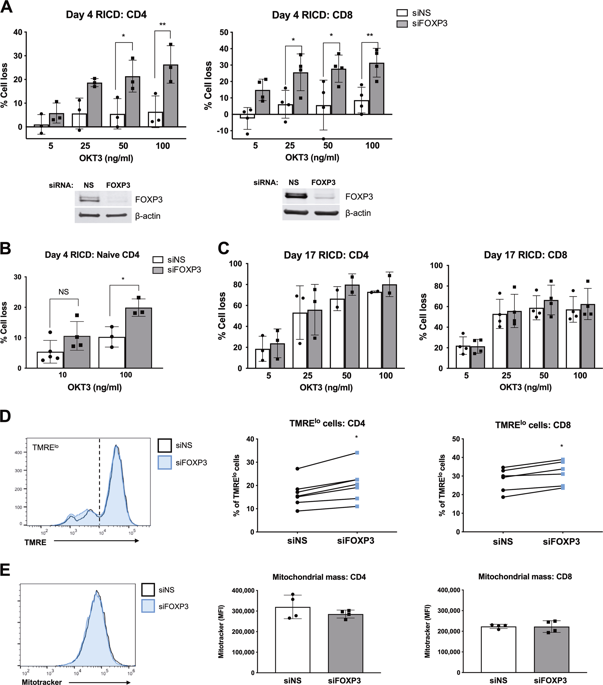
The adaptive immune response relies on specific apoptotic programs to maintain homeostasis. Conventional effector T cell (Tcon) expansion is constrained by both forkhead box P3 (FOXP3)+-regulatory T cells (Tregs) and restimulation-induced cell death (RICD), a propriocidal apoptosis pathway triggered by repeated stimulation through the T-cell receptor (TCR). Constitutive FOXP3 expression protects Tregs from RICD by suppressing SLAM-associated protein (SAP), a key adaptor protein that amplifies TCR signaling strength. The role of transient FOXP3 induction in activated human CD4 and CD8 Tcons remains unresolved, but its expression is inversely correlated with acquired RICD sensitivity. Here, we describe a novel role for FOXP3 in protecting human Tcons from premature RICD during expansion. Unlike FOXP3-mediated protection from RICD in Tregs, FOXP3 protects Tcons through a distinct mechanism requiring de novo transcription that does not require SAP suppression. Transcriptome profiling and functional analyses of expanding Tcons revealed that FOXP3 enhances expression of the SLAM family receptor CD48, which in turn sustains basal autophagy and suppresses pro-apoptotic p53 signaling. Both CD48 and FOXP3 expression reduced p53 accumulation upon TCR restimulation. Furthermore, silencing FOXP3 expression or blocking CD48 decreased the mitochondrial membrane potential in expanding Tcons with a concomitant reduction in basal autophagy. Our findings suggest that FOXP3 governs a distinct transcriptional program in early-stage effector Tcons that maintains RICD resistance via CD48-dependent protective autophagy and p53 suppression.
中文翻译:

FOXP3 保护传统人类 T 细胞免受过早再刺激诱导的细胞死亡
适应性免疫反应依赖于特定的凋亡程序来维持体内平衡。传统的效应 T 细胞 (Tcon) 扩展受到两个叉头盒 P3 (FOXP3) +- 调节性 T 细胞 (Tregs) 和再刺激诱导的细胞死亡 (RICD),这是一种通过 T 细胞受体 (TCR) 重复刺激触发的固有杀伤细胞凋亡途径。组成型 FOXP3 表达通过抑制 SLAM 相关蛋白 (SAP)(一种放大 TCR 信号强度的关键衔接蛋白)来保护 Treg 免受 RICD。瞬时 FOXP3 诱导在激活的人类 CD4 和 CD8 Tcon 中的作用仍未解决,但其表达与获得性 RICD 敏感性呈负相关。在这里,我们描述了 FOXP3 在扩展过程中保护人类 Tcon 免受过早 RICD 的新作用。与 FOXP3 介导的 Tregs RICD 保护不同,FOXP3 通过不需要 SAP 抑制的需要从头转录的独特机制保护 Tcon。扩增 Tcon 的转录组分析和功能分析表明,FOXP3 增强了 SLAM 家族受体 CD48 的表达,这反过来又维持了基础自噬并抑制了促凋亡 p53 信号传导。CD48 和 FOXP3 表达都减少了 TCR 再刺激后 p53 的积累。此外,沉默 FOXP3 表达或阻断 CD48 降低了扩大 Tcon 的线粒体膜电位,同时降低了基础自噬。我们的研究结果表明,FOXP3 在早期效应 Tcon 中控制着一个独特的转录程序,该程序通过 CD48 依赖性保护性自噬和 p53 抑制来维持 RICD 抗性。CD48 和 FOXP3 表达都减少了 TCR 再刺激后 p53 的积累。此外,沉默 FOXP3 表达或阻断 CD48 降低了扩大 Tcon 的线粒体膜电位,同时降低了基础自噬。我们的研究结果表明,FOXP3 在早期效应 Tcon 中控制着一个独特的转录程序,该程序通过 CD48 依赖性保护性自噬和 p53 抑制来维持 RICD 抗性。CD48 和 FOXP3 表达都减少了 TCR 再刺激后 p53 的积累。此外,沉默 FOXP3 表达或阻断 CD48 降低了扩大 Tcon 的线粒体膜电位,同时降低了基础自噬。我们的研究结果表明,FOXP3 在早期效应 Tcon 中控制着一个独特的转录程序,该程序通过 CD48 依赖性保护性自噬和 p53 抑制来维持 RICD 抗性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号