当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
2-O-N-Benzylcarbamoyl as a Protecting Group To Promote β-Selective Glycosylation and Its Applications in the Stereoselective Synthesis of Oligosaccharides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.joc.8b00047 Yin-Jen Lu,Yen-Hsun Lai,You-Yu Lin,Yi-Chi Wang,Pi-Hui Liang
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.joc.8b00047 Yin-Jen Lu,Yen-Hsun Lai,You-Yu Lin,Yi-Chi Wang,Pi-Hui Liang
|
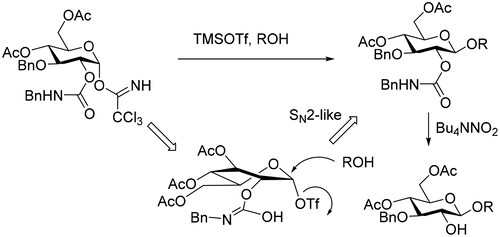
This study examines the utility of the N-benzylcarbamoyl (BnCar) protecting group in glycosylation reactions of the parent O-2 protected carbohydrate donor. It was found that the BnCar group imparted exclusively β-selectivity with primary and secondary alcohols. A mechanistic study revealed the activated intermediate to be the glycosyl triflate in a skew conformation, which results in β-selective glycosylation via an SN2-like pathway. The BnCar group can be readily cleaved using tetrabutylammonium nitrite, without affecting ester and ether protecting groups. Taken together, these results show BnCar to be useful for the synthesis of complex oligosaccharides, an undertaking that requires delicate chemical differentiation of various protecting groups.
中文翻译:

2- O - N-苄基氨基甲酰基作为保护基团,促进β-选择性糖基化反应及其在寡糖立体选择性合成中的应用
这项研究研究了N-苄氨基甲酰基(BnCar)保护基在亲本O -2保护的碳水化合物供体的糖基化反应中的作用。发现BnCar基团仅赋予伯醇和仲醇β-选择性。一项机理研究表明,活化的中间体为偏构型的三氟甲磺酸糖基酯,可通过类似S N 2的途径导致β选择性糖基化。BnCar基团可以使用亚硝酸四丁铵容易地裂解,而不会影响酯和醚保护基。综上所述,这些结果表明BnCar可用于合成复杂的低聚糖,这项工作需要对各种保护基进行精细的化学区分。
更新日期:2018-03-07
中文翻译:

2- O - N-苄基氨基甲酰基作为保护基团,促进β-选择性糖基化反应及其在寡糖立体选择性合成中的应用
这项研究研究了N-苄氨基甲酰基(BnCar)保护基在亲本O -2保护的碳水化合物供体的糖基化反应中的作用。发现BnCar基团仅赋予伯醇和仲醇β-选择性。一项机理研究表明,活化的中间体为偏构型的三氟甲磺酸糖基酯,可通过类似S N 2的途径导致β选择性糖基化。BnCar基团可以使用亚硝酸四丁铵容易地裂解,而不会影响酯和醚保护基。综上所述,这些结果表明BnCar可用于合成复杂的低聚糖,这项工作需要对各种保护基进行精细的化学区分。








































 京公网安备 11010802027423号
京公网安备 11010802027423号