当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Pyridine N-Oxidation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-10-26 , DOI: 10.1021/jacs.9b10414 Sheng-Ying Hsieh 1 , Yu Tang 1 , Simone Crotti 1 , Elizabeth A Stone 1 , Scott J Miller 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-10-26 , DOI: 10.1021/jacs.9b10414 Sheng-Ying Hsieh 1 , Yu Tang 1 , Simone Crotti 1 , Elizabeth A Stone 1 , Scott J Miller 1
Affiliation
|
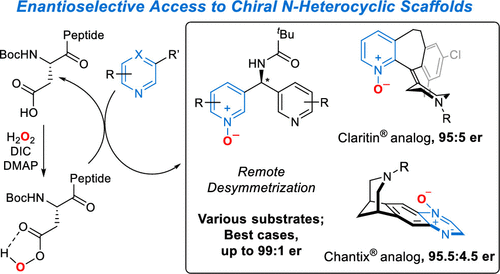
The catalytic, enantioselective N-oxidation of substituted pyridines is described. The approach is predicated on a biomolecule-inspired catalytic cycle wherein high levels of asymmetric induction are provided by aspartic acid-containing peptides as the aspartyl side chain shuttles between free acid and peracid forms. Desymmetrizations of bis(pyridine) substrates bearing a remote pro-stereogenic center substituted with a group capable of hydrogen bonding to the catalyst are demonstrated. Our approach presents a new entry into chiral pyridine frameworks in a heterocycle-rich molecular environment. Representative functionalizations of the enantioenriched pyridine N-oxides further document the utility of this approach. Demonstration of the asymmetric N-oxidation in two venerable drug-like scaffolds, Loratadine and Varenicline, show the likely generality of the method for highly variable and distinct chiral environments, while also reveal-ing that the approach is applicable to both pyridines and 1,4-pyrazines.
中文翻译:

催化对映选择性吡啶 N-氧化
描述了取代吡啶的催化、对映选择性 N-氧化反应。该方法基于生物分子启发的催化循环,其中当天冬氨酰侧链在游离酸和过酸形式之间穿梭时,含天冬氨酸的肽提供高水平的不对称诱导。证明了带有被能够与催化剂形成氢键的基团取代的远程前立体中心的双(吡啶)底物的去对称化。我们的方法为富含杂环的分子环境中的手性吡啶框架提供了新的途径。对映体富集的吡啶 N-氧化物的代表性官能化进一步证明了该方法的实用性。两种古老的类药物支架(氯雷他定和伐尼克兰)中的不对称 N-氧化证明表明该方法在高度可变和独特的手性环境中可能具有普遍性,同时也揭示了该方法适用于吡啶和 1, 4-吡嗪类。
更新日期:2019-10-26
中文翻译:

催化对映选择性吡啶 N-氧化
描述了取代吡啶的催化、对映选择性 N-氧化反应。该方法基于生物分子启发的催化循环,其中当天冬氨酰侧链在游离酸和过酸形式之间穿梭时,含天冬氨酸的肽提供高水平的不对称诱导。证明了带有被能够与催化剂形成氢键的基团取代的远程前立体中心的双(吡啶)底物的去对称化。我们的方法为富含杂环的分子环境中的手性吡啶框架提供了新的途径。对映体富集的吡啶 N-氧化物的代表性官能化进一步证明了该方法的实用性。两种古老的类药物支架(氯雷他定和伐尼克兰)中的不对称 N-氧化证明表明该方法在高度可变和独特的手性环境中可能具有普遍性,同时也揭示了该方法适用于吡啶和 1, 4-吡嗪类。






























 京公网安备 11010802027423号
京公网安备 11010802027423号