当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh-Catalyzed Hydroformylation of 1,3-Butadiene and Pent-4-enal to Adipaldehyde in CO2-Expanded Media
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-11-08 , DOI: 10.1021/acs.iecr.9b05184 Maria-José Tenorio 1 , Raghunath V. Chaudhari 1, 2 , Bala Subramaniam 1, 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-11-08 , DOI: 10.1021/acs.iecr.9b05184 Maria-José Tenorio 1 , Raghunath V. Chaudhari 1, 2 , Bala Subramaniam 1, 2
Affiliation
|
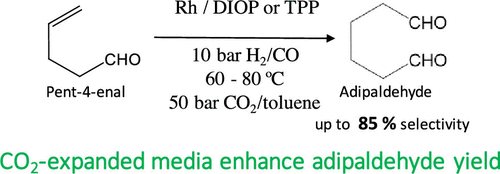
The homogeneous hydroformylation of pent-4-enal, the preferred aldehyde intermediate from 1,3-butadiene hydroformylation, was systematically investigated with Rh catalyst complexes in neat and CO2-expanded toluene media at 40–80 °C, syngas partial pressures ranging from 5–50 bar, and different ligand/Rh ratios. At similar operating conditions, the TOFs are generally greater with Rh/DIOP relative to a Rh/TPP catalyst. On both catalyst complexes, the chemoselectivity toward the dialdehydes ranges from 75%–100%, with the maximum adipaldehyde selectivity reaching approximately 75% (n/i ∼ 3) at 60 °C, 10 bar syngas, and molar DIOP/Rh ratio of 2.5. By using CO2-expanded toluene, the regioselectivity toward the adipaldehyde (desired product), and therefore its yield, is significantly enhanced. Interestingly, even with the simple Rh/TPP catalyst complex, adipaldehyde selectivity of up to 85% (n/i ∼ 5.6) is achieved at 60 °C, 10 bar syngas, and 50 bar CO2. The beneficial effects of CO2-expanded media are attributed to the facile tunability of the H2/CO ratio in such a phase with a fixed syngas feed composition. This approach to accelerate pent-4-enal hydroformylation to form adipaldehyde could also help in overcoming equilibrium limitations typically associated with the catalytic isomerization of pent-3-enal (the dominant product from 1,3-butadiene hydroformylation) to pent-4-enal (the preferred isomer).
中文翻译:

在CO 2膨胀介质中Rh催化1,3-丁二烯和Pent-4-烯醛的羰基化为己二醛
在40-80°C的纯净和CO 2膨胀的甲苯介质中,使用Rh催化剂配合物,在40-80°C,合成气分压范围为5–50 bar,以及不同的配体/ Rh比。在相似的操作条件下,相对于Rh / TPP催化剂,Rh / DIOP的TOF通常更大。在两种催化剂配合物上,对二醛的化学选择性范围为75%–100%,在60°C,10 bar合成气和DIOP / Rh摩尔比为60°C时,最大的己二醛选择性达到约75%(n / i〜3)。 2.5。通过使用CO 2在甲苯膨胀后,对己二醛(所需产物)的区域选择性大大提高。有趣的是,即使使用简单的Rh / TPP催化剂配合物,在60°C,10 bar合成气和50 bar CO 2的条件下,对己二醛的选择性仍可达到85%(n / i〜5.6)。CO 2膨胀介质的有益作用归因于H 2的易调谐性具有固定的合成气进料组成的这样的相中的/ CO比。这种加速戊4-烯醛加氢甲酰化形成己二醛的方法还可以帮助克服通常与戊3-烯醛(1,3-丁二烯加氢甲酰化的主要产物)催化异构化为戊4-烯醛相关的平衡限制。 (优选的异构体)。
更新日期:2019-11-08
中文翻译:

在CO 2膨胀介质中Rh催化1,3-丁二烯和Pent-4-烯醛的羰基化为己二醛
在40-80°C的纯净和CO 2膨胀的甲苯介质中,使用Rh催化剂配合物,在40-80°C,合成气分压范围为5–50 bar,以及不同的配体/ Rh比。在相似的操作条件下,相对于Rh / TPP催化剂,Rh / DIOP的TOF通常更大。在两种催化剂配合物上,对二醛的化学选择性范围为75%–100%,在60°C,10 bar合成气和DIOP / Rh摩尔比为60°C时,最大的己二醛选择性达到约75%(n / i〜3)。 2.5。通过使用CO 2在甲苯膨胀后,对己二醛(所需产物)的区域选择性大大提高。有趣的是,即使使用简单的Rh / TPP催化剂配合物,在60°C,10 bar合成气和50 bar CO 2的条件下,对己二醛的选择性仍可达到85%(n / i〜5.6)。CO 2膨胀介质的有益作用归因于H 2的易调谐性具有固定的合成气进料组成的这样的相中的/ CO比。这种加速戊4-烯醛加氢甲酰化形成己二醛的方法还可以帮助克服通常与戊3-烯醛(1,3-丁二烯加氢甲酰化的主要产物)催化异构化为戊4-烯醛相关的平衡限制。 (优选的异构体)。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号