当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Endohedral Metallofullerene M2@C80: A New Class of Magnetic Superhalogen
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-10-25 00:00:00 , DOI: 10.1021/acs.jpcc.9b08340 Yuhang Jiang 1 , Zisheng Li 1 , Jinying Zhang 2 , Zhiyong Wang 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-10-25 00:00:00 , DOI: 10.1021/acs.jpcc.9b08340 Yuhang Jiang 1 , Zisheng Li 1 , Jinying Zhang 2 , Zhiyong Wang 1
Affiliation
|
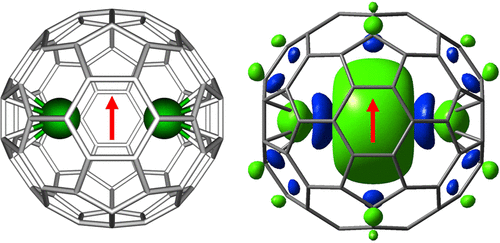
Superhalogen is a special class of atomic clusters whose electron affinity is larger than that of chlorine atom, which has the largest electron affinity in the periodic table of elements. In this paper, we report the fabrication of a new class of magnetic superhalogen by encapsulating two rare-earth metal atoms into the Ih symmetrical C80 fullerene. The as-produced metallofullerene has an electronic configuration of M25+@C805–. The addition of an extra electron to the molecule leads to the formation of a highly stable structure M25+@C806–. Density functional theory calculations reveal that M2@C80 has a larger electron affinity than the chlorine atom. Thus, M2@C80 can be classified as superhalogen. The magnetic properties of the M2@C80 superhalogens are associated with the single-electron metal–metal molecular orbital and the partially filled f electron shell of the metal atoms. Electron spin resonance measurement discloses the paramagnetic character of Y2@C80– and the strong coupling effect between the unpaired electron and the Y nuclei. The outer fullerene cage provides a suitable environment for protecting the unconventional valence and spin states of the inner metal atoms. A variety of superhalogens with distinct magnetic properties can be obtained by encapsulating different lanthanide metal atoms into the C80 fullerene. Such superhalogens can serve as building blocks of materials with tailored magnetic properties.
中文翻译:

内表面金属富勒烯M2 @ C80:新型的磁性超级卤素
超卤素是一类特殊的原子团簇,其电子亲和力大于氯原子,而氯原子在元素周期表中的电子亲和力最大。在本文中,我们报告了通过将两个稀土金属原子封装到I h对称的C 80富勒烯中来制造一类新型的磁性超级卤素的方法。所生产的金属富勒烯的电子构型为M 2 5+ @C 80 5–。向分子中添加额外的电子会导致形成高度稳定的结构M 2 5+ @C 80 6–。密度泛函理论计算表明M 2@C 80具有比氯原子更大的电子亲和力。因此,M 2 C 80可以归类为超卤素。M 2 @C 80超级卤素的磁性与单电子金属-金属分子轨道和部分填充的金属原子的f电子壳有关。电子自旋共振测量揭示了Y 2 @C 80 –的顺磁特性以及未成对电子与Y原子核之间的强耦合效应。外部富勒烯笼为保护内部金属原子的非常规化合价和自旋态提供了合适的环境。通过将不同的镧系元素金属原子封装到C 80富勒烯中,可以获得具有不同磁性的各种超卤素。这样的超卤素可以用作具有定制的磁性的材料的构造块。
更新日期:2019-10-25
中文翻译:

内表面金属富勒烯M2 @ C80:新型的磁性超级卤素
超卤素是一类特殊的原子团簇,其电子亲和力大于氯原子,而氯原子在元素周期表中的电子亲和力最大。在本文中,我们报告了通过将两个稀土金属原子封装到I h对称的C 80富勒烯中来制造一类新型的磁性超级卤素的方法。所生产的金属富勒烯的电子构型为M 2 5+ @C 80 5–。向分子中添加额外的电子会导致形成高度稳定的结构M 2 5+ @C 80 6–。密度泛函理论计算表明M 2@C 80具有比氯原子更大的电子亲和力。因此,M 2 C 80可以归类为超卤素。M 2 @C 80超级卤素的磁性与单电子金属-金属分子轨道和部分填充的金属原子的f电子壳有关。电子自旋共振测量揭示了Y 2 @C 80 –的顺磁特性以及未成对电子与Y原子核之间的强耦合效应。外部富勒烯笼为保护内部金属原子的非常规化合价和自旋态提供了合适的环境。通过将不同的镧系元素金属原子封装到C 80富勒烯中,可以获得具有不同磁性的各种超卤素。这样的超卤素可以用作具有定制的磁性的材料的构造块。






























 京公网安备 11010802027423号
京公网安备 11010802027423号