当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ACTL6A regulates follicle-stimulating hormone-driven glycolysis in ovarian cancer cells via PGK1.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-10-24 , DOI: 10.1038/s41419-019-2050-y Jiawen Zhang 1 , Jing Zhang 2, 3 , Yingze Wei 4 , Qingxian Li 5 , Qingying Wang 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-10-24 , DOI: 10.1038/s41419-019-2050-y Jiawen Zhang 1 , Jing Zhang 2, 3 , Yingze Wei 4 , Qingxian Li 5 , Qingying Wang 1
Affiliation
|
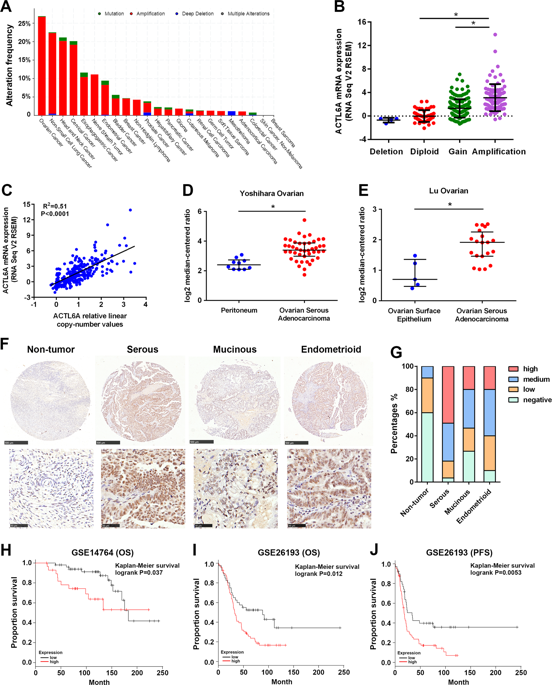
Enhanced glycolysis has been identified as a hallmark of cancer. As a novel oncogene, ACTL6A is aberrantly amplified in several types of human cancers and has been shown to regulate tumor growth and progression. However, the roles of ACTL6A in the development of ovarian cancer and the regulation of cancer glucose metabolism are mostly unknown. Here we show that ACTL6A is overexpressed in ovarian cancers compared with adjacent non-tumor tissues, and that ACTL6A overexpression correlates with poor prognosis. Silencing of ACTL6A in vitro inhibits proliferation, clonal growth, and migration, and decreases glucose utilization, lactate production, and pyruvate levels of ovarian cancer cells. We found a positive correlation between ACTL6A and PGK1 expression in ovarian cancer tissues. Enforced ACTL6A expression increased PGK1 expression, whereas knockdown of ACTL6A had the opposite effect. Altered ACTL6A expression inhibits the tumorigenicity of ovarian cancer cells in vivo by downregulating PGK1. In addition, the expression of ACTL6A is regulated by follicle-stimulating hormone (FSH) stimulation via PI3K/AKT pathway. Importantly, ACTL6A regulates FSH-enhanced glycolysis in ovarian cancer. Taken together, our findings highlight the critical role of ACTL6A in ovarian cancer development and identify its contribution to glucose metabolism of cancer cells.
中文翻译:

ACTL6A通过PGK1调节卵巢癌细胞中促卵泡激素驱动的糖酵解。
糖酵解的增强已被确认为癌症的标志。作为一种新型致癌基因,ACTL6A在几种类型的人类癌症中异常扩增,并已显示出可调节肿瘤的生长和进程。然而,ACTL6A在卵巢癌的发展中的作用以及癌糖代谢的调节大多是未知的。在这里,我们显示ACTL6A在卵巢癌中与邻近的非肿瘤组织相比过表达,并且ACTL6A的过表达与不良预后相关。体外使ACTL6A沉默可抑制卵巢癌细胞的增殖,克隆生长和迁移,并降低葡萄糖利用率,乳酸生成和丙酮酸水平。我们发现卵巢癌组织中ACTL6A和PGK1表达之间呈正相关。强制ACTL6A表达增加PGK1表达,而敲低ACTL6A具有相反的效果。改变的ACTL6A表达通过下调PGK1体内抑制卵巢癌细胞的致瘤性。此外,通过PI3K / AKT途径的卵泡刺激素(FSH)刺激来调节ACTL6A的表达。重要的是,ACTL6A调节卵巢癌中FSH增强的糖酵解。综上所述,我们的发现突出了ACTL6A在卵巢癌发展中的关键作用,并确定了其对癌细胞葡萄糖代谢的贡献。ACTL6A调节卵巢癌中FSH增强的糖酵解。综上所述,我们的发现突出了ACTL6A在卵巢癌发展中的关键作用,并确定了其对癌细胞葡萄糖代谢的贡献。ACTL6A调节卵巢癌中FSH增强的糖酵解。综上所述,我们的发现突出了ACTL6A在卵巢癌发展中的关键作用,并确定了其对癌细胞葡萄糖代谢的贡献。
更新日期:2019-10-25
中文翻译:

ACTL6A通过PGK1调节卵巢癌细胞中促卵泡激素驱动的糖酵解。
糖酵解的增强已被确认为癌症的标志。作为一种新型致癌基因,ACTL6A在几种类型的人类癌症中异常扩增,并已显示出可调节肿瘤的生长和进程。然而,ACTL6A在卵巢癌的发展中的作用以及癌糖代谢的调节大多是未知的。在这里,我们显示ACTL6A在卵巢癌中与邻近的非肿瘤组织相比过表达,并且ACTL6A的过表达与不良预后相关。体外使ACTL6A沉默可抑制卵巢癌细胞的增殖,克隆生长和迁移,并降低葡萄糖利用率,乳酸生成和丙酮酸水平。我们发现卵巢癌组织中ACTL6A和PGK1表达之间呈正相关。强制ACTL6A表达增加PGK1表达,而敲低ACTL6A具有相反的效果。改变的ACTL6A表达通过下调PGK1体内抑制卵巢癌细胞的致瘤性。此外,通过PI3K / AKT途径的卵泡刺激素(FSH)刺激来调节ACTL6A的表达。重要的是,ACTL6A调节卵巢癌中FSH增强的糖酵解。综上所述,我们的发现突出了ACTL6A在卵巢癌发展中的关键作用,并确定了其对癌细胞葡萄糖代谢的贡献。ACTL6A调节卵巢癌中FSH增强的糖酵解。综上所述,我们的发现突出了ACTL6A在卵巢癌发展中的关键作用,并确定了其对癌细胞葡萄糖代谢的贡献。ACTL6A调节卵巢癌中FSH增强的糖酵解。综上所述,我们的发现突出了ACTL6A在卵巢癌发展中的关键作用,并确定了其对癌细胞葡萄糖代谢的贡献。































 京公网安备 11010802027423号
京公网安备 11010802027423号