Theranostics ( IF 12.4 ) Pub Date : 2020-01-01 , DOI: 10.7150/thno.35841 Lin-Yuan Zhang 1 , Jiaji Pan 1 , Muyassar Mamtilahun 1 , Yuan Zhu 1 , Liping Wang 1 , Ashwin Venkatesh 2 , Rubing Shi 1 , Xuanqiang Tu 1 , Kunlin Jin 3 , Yongting Wang 1 , Zhijun Zhang 1 , Guo-Yuan Yang 1
|
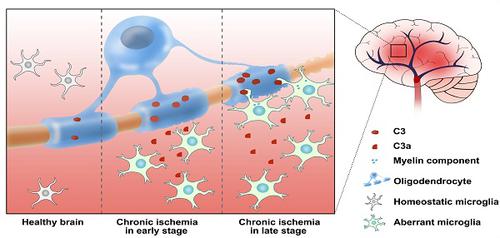
Microglial activation participates in white matter injury after cerebral hypoperfusion. However, the underlying mechanism is unclear. Here, we explore whether activated microglia aggravate white matter injury via complement C3-C3aR pathway after chronic cerebral hypoperfusion.
Methods: Adult male Sprague-Dawley rats (n = 80) underwent bilateral common carotid artery occlusion for 7, 14, and 28 days. Cerebral vessel density and blood flow were examined by synchrotron radiation angiography and three-dimensional arterial spin labeling. Neurobehavioral assessments, CLARITY imaging, and immunohistochemistry were performed to evaluate activation of microglia and C3-C3aR pathway. Furthermore, C3aR knockout mice were used to establish the causal relationship of C3-C3aR signaling on microglia activation and white matter injury after hypoperfusion.
Results: Cerebral vessel density and blood flow were reduced after hypoperfusion (p<0.05). Spatial learning and memory deficits and white matter injury were shown (p<0.05). These impairments were correlated with aberrant microglia activation and an increase in the number of reactive microglia adhering to and phagocytosed myelin in the hypoperfusion group (p<0.05), which were accompanied by the up-regulation of complement C3 and its receptors C3aR (p<0.05). Genetic deletion of C3ar1 significantly inhibited aberrant microglial activation and reversed white matter injury after hypoperfusion (p<0.05). Furthermore, the C3aR antagonist SB290157 decreased the number of microglia adhering to myelin (p<0.05), attenuated white matter injury and cognitive deficits in chronic hypoperfusion rats (p<0.05).
Conclusions: Our results demonstrated that aberrant activated microglia aggravate white matter injury via C3-C3aR pathway during chronic hypoperfusion. These findings indicate C3aR plays a critical role in mediating neuroinflammation and white matter injury through aberrant microglia activation, which provides a novel therapeutic target for the small vessel disease and vascular dementia.
中文翻译:

小胶质细胞低灌注后通过补体C3 / C3aR途径加剧白质损伤。
小胶质细胞激活参与脑灌注不足后的白质损伤。但是,其潜在机制尚不清楚。在这里,我们探讨慢性脑灌注不足后活化的小胶质细胞是否通过补体C3-C3aR途径加重白质损伤。
方法:成年雄性Sprague-Dawley大鼠(n = 80)进行双侧颈总动脉闭塞7、14和28天。通过同步辐射血管造影和三维动脉自旋标记检查脑血管密度和血流量。进行神经行为评估,清晰度成像和免疫组织化学,以评估小胶质细胞和C3-C3aR途径的激活。此外,使用C3aR基因敲除小鼠建立C3-C3aR信号传导与低灌注后小胶质细胞激活和白质损伤的因果关系。
结果:灌注不足后脑血管密度和血流量降低(p < 0.05)。显示了空间学习和记忆缺陷以及白质损伤(p < 0.05)。这些损伤与低灌注组中异常的小胶质细胞活化以及粘附和吞噬髓磷脂的反应性小胶质细胞数量增加相关(p < 0.05),同时伴有补体C3及其受体C3aR的上调(p < 0.05)。C3ar1的基因缺失显着抑制了低灌注后小胶质细胞的异常激活和白质损伤的逆转(p <0.05)。此外,在慢性低灌注大鼠中,C3aR拮抗剂SB290157减少了粘附于髓磷脂的小胶质细胞的数量(p < 0.05),减轻了白质损伤和认知缺陷(p < 0.05)。
结论:我们的结果表明,在慢性低灌注期间,异常激活的小胶质细胞会通过C3-C3aR途径加重白质损伤。这些发现表明,C3aR通过异常的小胶质细胞活化在介导神经炎症和白质损伤中起关键作用,这为小血管疾病和血管性痴呆症提供了新的治疗靶标。































 京公网安备 11010802027423号
京公网安备 11010802027423号