Molecular Immunology ( IF 3.2 ) Pub Date : 2018-03-07 , DOI: 10.1016/j.molimm.2018.02.016
Kelly R. Rhodes , Jordan J. Green
|
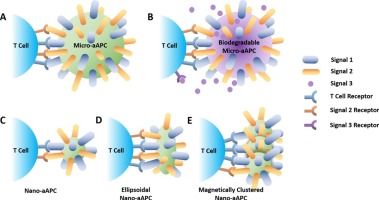
Exciting developments in cancer nanomedicine include the engineering of nanocarriers to deliver drugs locally to tumors, increasing efficacy and reducing off-target toxicity associated with chemotherapies. Despite nanocarrier advances, metastatic cancer remains challenging to treat due to barriers that prevent nanoparticles from gaining access to remote, dispersed, and poorly vascularized metastatic tumors. Instead of relying on nanoparticles to directly destroy every tumor cell, immunotherapeutic approaches target immune cells to train them to recognize and destroy tumor cells, which, due to the amplification and specificity of an adaptive immune response, may be a more effective approach to treating metastatic cancer. One novel technology for cancer immunotherapy is the artificial antigen presenting cell (aAPC), a micro- or nanoparticle-based system that mimics an antigen presenting cell by presenting important signal proteins to T cells to activate them against cancer. Signal 1 molecules target the T cell receptor and facilitate antigen recognition by T cells, signal 2 molecules provide costimulation essential for T cell activation, and signal 3 consists of secreted cues that further stimulate T cells. Classic microscale aAPCs present signal 1 and 2 molecules on their surface, and biodegradable polymeric aAPCs offer the additional capability of releasing signal 3 cytokines and costimulatory molecules that modulate the T cell response. Although particles of approximately 5–10 μm in diameter may be considered the optimal size of an aAPC for ex vivo cellular expansion, nanoscale aAPCs have demonstrated superior in vivo pharmacokinetic properties and are more suitable for systemic injection. As sufficient surface contact between T cells and aAPCs is essential for activation, nano-aAPCs with microscale contact surface areas have been created through engineering approaches such as shape manipulation and nanoparticle clustering. These design strategies have demonstrated greatly enhanced efficacy of nano-aAPCs, endowing nano-aAPCs with the potential to be among the next generation of cancer nanomedicines.
中文翻译:

用于癌症免疫治疗的纳米级人工抗原呈递细胞
癌症纳米医学的激动人心的发展包括纳米载体的工程化,以将药物局部递送至肿瘤,提高功效并减少与化学疗法相关的脱靶毒性。尽管纳米载体取得了进步,但转移性癌症的治疗仍然面临挑战,原因是其阻碍了纳米颗粒无法进入较远,分散且血管化程度差的转移性肿瘤。免疫疗法不是依靠纳米粒子直接破坏每个肿瘤细胞,而是针对免疫细胞来训练它们识别和破坏肿瘤细胞,由于适应性免疫应答的扩增和特异性,它可能是治疗转移性疾病的更有效方法。癌症。一种用于癌症免疫疗法的新技术是人工抗原呈递细胞(aAPC),一种基于微米或纳米粒子的系统,通过将重要的信号蛋白呈递给T细胞来激活它们来抵抗癌症,从而模仿抗原呈递细胞。信号1分子靶向T细胞受体并促进T细胞识别抗原,信号2分子提供了T细胞活化必不可少的共刺激信号,信号3则由进一步刺激T细胞的分泌线索组成。经典的微型aAPC在其表面上存在信号1和2分子,可生物降解的聚合aAPC提供释放信号3细胞因子和共刺激分子的额外功能,这些分子调节T细胞反应。尽管可以认为直径约5–10μm的颗粒的aAPC的最佳尺寸 信号1分子靶向T细胞受体并促进T细胞识别抗原,信号2分子提供了T细胞活化必不可少的共刺激信号,信号3则由进一步刺激T细胞的分泌线索组成。经典的微型aAPC在其表面上存在信号1和2分子,可生物降解的聚合aAPC提供释放信号3细胞因子和共刺激分子的额外功能,这些分子调节T细胞反应。尽管可以认为直径约5–10μm的颗粒的aAPC的最佳尺寸 信号1分子靶向T细胞受体并促进T细胞识别抗原,信号2分子提供了T细胞活化必不可少的共刺激信号,信号3则由进一步刺激T细胞的分泌线索组成。经典的微型aAPC在其表面上存在信号1和2分子,可生物降解的聚合aAPC提供释放信号3细胞因子和共刺激分子的额外功能,这些分子调节T细胞反应。尽管可以认为直径约5–10μm的颗粒的aAPC的最佳尺寸 可生物降解的聚合物aAPC具有释放信号3细胞因子和调节T细胞反应的共刺激分子的附加功能。尽管可以认为直径约5–10μm的颗粒的aAPC的最佳尺寸 可生物降解的聚合物aAPC具有释放信号3细胞因子和调节T细胞反应的共刺激分子的附加功能。尽管可以认为直径约5–10μm的颗粒的aAPC的最佳尺寸在体外细胞扩增中,纳米级aAPCs已显示出优异的体内药代动力学特性,更适合全身注射。由于T细胞和aAPC之间足够的表面接触对于激活至关重要,因此已经通过诸如形状处理和纳米粒子簇集等工程方法创建了具有微型接触表面积的纳米aAPC。这些设计策略已经证明了纳米aAPC的功效大大增强,赋予了纳米aAPC潜在的下一代癌症纳米药物的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号