当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ZnO-NP assisted synthesis of fluorescent β-carboline C-1 tethered benzimidazole/benzothiazole/benzoxazole derivatives and assessment of their photophysical properties
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2019-10-24 , DOI: 10.1039/c9nj04256c Vipin Kumar 1, 2, 3, 4 , Dharmender Singh 1, 2, 3, 4 , Avijit Kumar Paul 1, 4, 5, 6 , Rahul Shrivastava 1, 4, 7 , Virender Singh 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2019-10-24 , DOI: 10.1039/c9nj04256c Vipin Kumar 1, 2, 3, 4 , Dharmender Singh 1, 2, 3, 4 , Avijit Kumar Paul 1, 4, 5, 6 , Rahul Shrivastava 1, 4, 7 , Virender Singh 1, 2, 3, 4
Affiliation
|
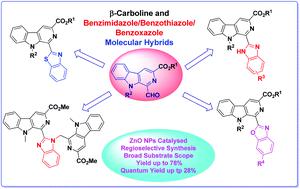
A facile transformation of 1-formyl β-carboline into fluorescent β-carboline C-1 tethered benzazole derivatives is described under the catalysis of ZnO nanoparticles. The reaction proceeded with the reaction of 1-formyl β-carboline and substituted o-diaminobenzene/2-aminobenzenethiol/2-aminophenol, which results in formation of a Schiff base, followed by an intramolecular cylization reaction to generate β-carboline linked benzimidazole, benzothiazole and benzoxazole derivatives. This appraoch displayed a wide substrate scope and high regioselectivity to yield the desired products in moderate to good yields. The photophysical properties of the synthesized derivatives were also evaluated and they exhibited excellent fluorescence properties. Among these β-carboline substituted azoles, the benzothiazole derivative displayed the maximum quantum yield (ΦF up to 28%).
中文翻译:

ZnO-NP辅助合成荧光β-咔啉C-1束缚苯并咪唑/苯并噻唑/苯并恶唑衍生物及其光物理性质
在ZnO纳米粒子的催化作用下,描述了1-甲酰基β-咔啉向荧光β-咔啉C-1系苯并恶唑衍生物的轻松转化。反应进行至1-甲酰基β-咔啉与取代的o-二氨基苯/ 2-氨基苯硫醇/ 2-氨基苯酚,导致形成席夫碱,然后进行分子内环化反应,生成β-咔啉连接的苯并咪唑,苯并噻唑和苯并恶唑衍生物。该方法显示了宽的底物范围和高的区域选择性,以中等至良好的产率产生了所需的产物。还评估了合成衍生物的光物理性质,它们表现出优异的荧光性质。在这些β咔啉取代的唑类,苯并噻唑衍生物显示的最大量子产率(Φ ˚F高达28%)。
更新日期:2019-10-24
中文翻译:

ZnO-NP辅助合成荧光β-咔啉C-1束缚苯并咪唑/苯并噻唑/苯并恶唑衍生物及其光物理性质
在ZnO纳米粒子的催化作用下,描述了1-甲酰基β-咔啉向荧光β-咔啉C-1系苯并恶唑衍生物的轻松转化。反应进行至1-甲酰基β-咔啉与取代的o-二氨基苯/ 2-氨基苯硫醇/ 2-氨基苯酚,导致形成席夫碱,然后进行分子内环化反应,生成β-咔啉连接的苯并咪唑,苯并噻唑和苯并恶唑衍生物。该方法显示了宽的底物范围和高的区域选择性,以中等至良好的产率产生了所需的产物。还评估了合成衍生物的光物理性质,它们表现出优异的荧光性质。在这些β咔啉取代的唑类,苯并噻唑衍生物显示的最大量子产率(Φ ˚F高达28%)。













































 京公网安备 11010802027423号
京公网安备 11010802027423号