当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Paired electrocatalytic hydrogenation and oxidation of 5-(hydroxymethyl)furfural for efficient production of biomass-derived monomers
Green Chemistry ( IF 9.3 ) Pub Date : 2019-10-23 , DOI: 10.1039/c9gc02264c
Xiaotong H. Chadderdon 1, 2, 3, 4 , David J. Chadderdon 1, 2, 3, 4 , Toni Pfennig 1, 2, 3, 4, 5 , Brent H. Shanks 1, 2, 3, 4, 5 , Wenzhen Li 1, 2, 3, 4, 6
Green Chemistry ( IF 9.3 ) Pub Date : 2019-10-23 , DOI: 10.1039/c9gc02264c
Xiaotong H. Chadderdon 1, 2, 3, 4 , David J. Chadderdon 1, 2, 3, 4 , Toni Pfennig 1, 2, 3, 4, 5 , Brent H. Shanks 1, 2, 3, 4, 5 , Wenzhen Li 1, 2, 3, 4, 6
Affiliation
|
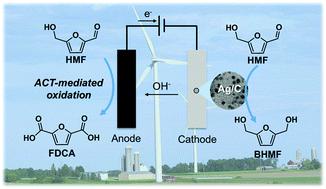
Electrochemical conversion of biomass-derived compounds is a promising route for sustainable chemical production. Herein, we report unprecedentedly high efficiency for conversion of 5-(hydroxymethyl)furfural (HMF) to biobased monomers by pairing HMF reduction and oxidation half-reactions in one electrochemical cell. Electrocatalytic hydrogenation of HMF to 2,5-bis(hydroxymethyl)furan (BHMF) was achieved under mild conditions using carbon-supported Ag nanoparticles (Ag/C) as the cathode catalyst. The competition between Ag-catalyzed HMF hydrogenation to BHMF and undesired HMF hydrodimerization and hydrogen evolution reactions was sensitive to cathode potential. Also, the carbon support material in Ag/C was active for HMF reduction at strongly cathodic potentials, leading to additional hydrodimerization and low BHMF selectivity. Accordingly, precise control of the cathode potential was implemented to achieve high BHMF selectivity and efficiency. In contrast, the selectivity of HMF oxidation facilitated by a homogeneous electrocatalyst, 4-acetamido-TEMPO (ACT, TEMPO = 2,2,6,6-tetramethylpiperidine-1-oxyl), together with an inexpensive carbon felt electrode, was insensitive to anode potential. Thus, it was feasible to conduct HMF hydrogenation to BHMF and oxidation to 2,5-furandicarboxylic acid (FDCA) in a single divided cell operated under cathode potential control. Electrocatalytic HMF conversion in the paired cell achieved high yields of BHMF and FDCA (85% and 98%, respectively) and a combined electron efficiency of 187%, corresponding to a nearly two-fold enhancement compared to the unpaired cells.
中文翻译:

5-(羟甲基)糠醛的配对电催化加氢和氧化以有效生产生物质衍生的单体
生物质衍生化合物的电化学转化是可持续化学生产的有希望的途径。在本文中,我们报道了通过在一个电化学电池中将HMF还原和氧化半反应配对,将5-(羟甲基)糠醛(HMF)转化为生物基单体的空前高效率。使用碳载Ag纳米粒子(Ag / C)作为阴极催化剂,在温和条件下实现了HMF的电催化加氢成2,5-双(羟甲基)呋喃(BHMF)。Ag催化的HMF加氢为BHMF与不希望的HMF加氢二聚反应和析氢反应之间的竞争对阴极电势敏感。同样,Ag / C中的碳载体材料在强阴极电势下也具有降低HMF的活性,从而导致额外的加氢二聚反应和低BHMF选择性。因此,实施了对阴极电势的精确控制,以实现高BHMF选择性和效率。相反,均质电催化剂4-乙酰氨基-TEMPO(ACT,TEMPO = 2,2,6,6-四甲基哌啶-1-氧基)以及廉价的碳毡电极对HMF氧化的选择性不敏感。阳极电位。因此,在一个在阴极电势控制下运行的单个分隔的电池中,将HMF氢化为BHMF并氧化为2,5-呋喃二甲酸(FDCA)是可行的。配对电池中的电催化HMF转化率很高,分别达到BHMF和FDCA的产率(分别为85%和98%),综合电子效率为187%,与未配对电池相比,提高了近两倍。
更新日期:2019-10-23
中文翻译:

5-(羟甲基)糠醛的配对电催化加氢和氧化以有效生产生物质衍生的单体
生物质衍生化合物的电化学转化是可持续化学生产的有希望的途径。在本文中,我们报道了通过在一个电化学电池中将HMF还原和氧化半反应配对,将5-(羟甲基)糠醛(HMF)转化为生物基单体的空前高效率。使用碳载Ag纳米粒子(Ag / C)作为阴极催化剂,在温和条件下实现了HMF的电催化加氢成2,5-双(羟甲基)呋喃(BHMF)。Ag催化的HMF加氢为BHMF与不希望的HMF加氢二聚反应和析氢反应之间的竞争对阴极电势敏感。同样,Ag / C中的碳载体材料在强阴极电势下也具有降低HMF的活性,从而导致额外的加氢二聚反应和低BHMF选择性。因此,实施了对阴极电势的精确控制,以实现高BHMF选择性和效率。相反,均质电催化剂4-乙酰氨基-TEMPO(ACT,TEMPO = 2,2,6,6-四甲基哌啶-1-氧基)以及廉价的碳毡电极对HMF氧化的选择性不敏感。阳极电位。因此,在一个在阴极电势控制下运行的单个分隔的电池中,将HMF氢化为BHMF并氧化为2,5-呋喃二甲酸(FDCA)是可行的。配对电池中的电催化HMF转化率很高,分别达到BHMF和FDCA的产率(分别为85%和98%),综合电子效率为187%,与未配对电池相比,提高了近两倍。































 京公网安备 11010802027423号
京公网安备 11010802027423号