Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis for enzymatic photocatalysis in chlorophyll biosynthesis
Nature ( IF 50.5 ) Pub Date : 2019-10-23 , DOI: 10.1038/s41586-019-1685-2 Shaowei Zhang 1 , Derren J Heyes 1 , Lingling Feng 2 , Wenli Sun 3 , Linus O Johannissen 1 , Huanting Liu 4 , Colin W Levy 1 , Xuemei Li 5 , Ji Yang 4 , Xiaolan Yu 4 , Min Lin 3 , Samantha J O Hardman 1 , Robin Hoeven 1 , Michiyo Sakuma 1 , Sam Hay 1 , David Leys 1 , Zihe Rao 5 , Aiwu Zhou 2 , Qi Cheng 3, 4, 6 , Nigel S Scrutton 1
Nature ( IF 50.5 ) Pub Date : 2019-10-23 , DOI: 10.1038/s41586-019-1685-2 Shaowei Zhang 1 , Derren J Heyes 1 , Lingling Feng 2 , Wenli Sun 3 , Linus O Johannissen 1 , Huanting Liu 4 , Colin W Levy 1 , Xuemei Li 5 , Ji Yang 4 , Xiaolan Yu 4 , Min Lin 3 , Samantha J O Hardman 1 , Robin Hoeven 1 , Michiyo Sakuma 1 , Sam Hay 1 , David Leys 1 , Zihe Rao 5 , Aiwu Zhou 2 , Qi Cheng 3, 4, 6 , Nigel S Scrutton 1
Affiliation
|
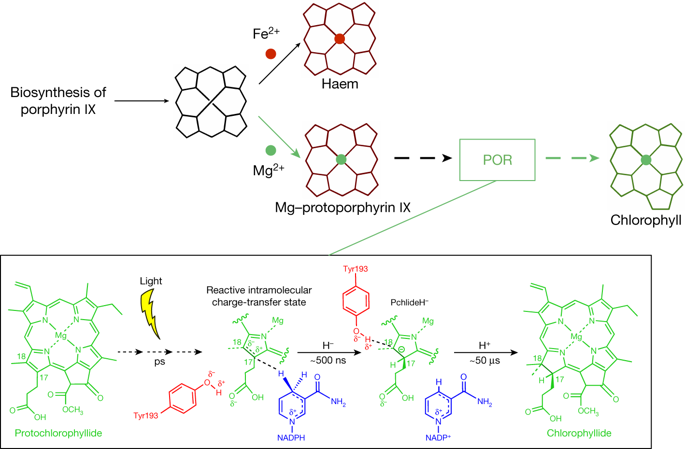
The enzyme protochlorophyllide oxidoreductase (POR) catalyses a light-dependent step in chlorophyll biosynthesis that is essential to photosynthesis and, ultimately, all life on Earth1–3. POR, which is one of three known light-dependent enzymes4,5, catalyses reduction of the photosensitizer and substrate protochlorophyllide to form the pigment chlorophyllide. Despite its biological importance, the structural basis for POR photocatalysis has remained unknown. Here we report crystal structures of cyanobacterial PORs from Thermosynechococcus elongatus and Synechocystis sp. in their free forms, and in complex with the nicotinamide coenzyme. Our structural models and simulations of the ternary protochlorophyllide–NADPH–POR complex identify multiple interactions in the POR active site that are important for protochlorophyllide binding, photosensitization and photochemical conversion to chlorophyllide. We demonstrate the importance of active-site architecture and protochlorophyllide structure in driving POR photochemistry in experiments using POR variants and protochlorophyllide analogues. These studies reveal how the POR active site facilitates light-driven reduction of protochlorophyllide by localized hydride transfer from NADPH and long-range proton transfer along structurally defined proton-transfer pathways.Crystal structures of cyanobacterial protochlorophyllide oxidoreductases reveal the basis of the photocatalytic activities of this enzyme, through the role of its active site in enabling the light-driven reduction of protochlorophyllide.
中文翻译:

叶绿素生物合成中酶促光催化的结构基础
原叶绿素氧化还原酶 (POR) 催化叶绿素生物合成中的一个依赖光的步骤,这对光合作用以及最终地球上的所有生命都是必不可少的 1-3。POR 是三种已知的光依赖性酶之一 4,5,可催化光敏剂和底物原叶绿素的还原,形成色素叶绿素。尽管具有生物学重要性,但 POR 光催化的结构基础仍然未知。在这里,我们报告了来自细长热聚球藻和集胞藻的蓝藻 POR 的晶体结构。以游离形式存在,并与烟酰胺辅酶复合。我们对三元原叶绿素内酯-NADPH-POR 复合物的结构模型和模拟确定了 POR 活性位点中的多种相互作用,这些相互作用对原叶绿素内酯结合很重要,光敏化和光化学转化为叶绿素。我们证明了活性位点结构和原叶绿素内酯结构在使用 POR 变体和原叶绿素类似物的实验中驱动 POR 光化学的重要性。这些研究揭示了 POR 活性位点如何通过 NADPH 的局部氢化物转移和沿着结构定义的质子转移途径的长程质子转移促进原叶绿素的光驱动还原。蓝藻原叶绿素内酯氧化还原酶的晶体结构揭示了这种光催化活性的基础酶,通过其活性位点在光驱动下还原原叶绿素的作用。我们证明了活性位点结构和原叶绿素内酯结构在使用 POR 变体和原叶绿素类似物的实验中驱动 POR 光化学的重要性。这些研究揭示了 POR 活性位点如何通过 NADPH 的局部氢化物转移和沿结构定义的质子转移途径的长程质子转移促进原叶绿素酸的光驱动还原。蓝藻原叶绿素内酯氧化还原酶的晶体结构揭示了这种光催化活性的基础酶,通过其活性位点在光驱动下还原原叶绿素的作用。我们证明了活性位点结构和原叶绿素内酯结构在使用 POR 变体和原叶绿素类似物的实验中驱动 POR 光化学的重要性。这些研究揭示了 POR 活性位点如何通过 NADPH 的局部氢化物转移和沿结构定义的质子转移途径的长程质子转移促进原叶绿素酸的光驱动还原。蓝藻原叶绿素内酯氧化还原酶的晶体结构揭示了这种光催化活性的基础酶,通过其活性位点在光驱动下还原原叶绿素的作用。
更新日期:2019-10-23
中文翻译:

叶绿素生物合成中酶促光催化的结构基础
原叶绿素氧化还原酶 (POR) 催化叶绿素生物合成中的一个依赖光的步骤,这对光合作用以及最终地球上的所有生命都是必不可少的 1-3。POR 是三种已知的光依赖性酶之一 4,5,可催化光敏剂和底物原叶绿素的还原,形成色素叶绿素。尽管具有生物学重要性,但 POR 光催化的结构基础仍然未知。在这里,我们报告了来自细长热聚球藻和集胞藻的蓝藻 POR 的晶体结构。以游离形式存在,并与烟酰胺辅酶复合。我们对三元原叶绿素内酯-NADPH-POR 复合物的结构模型和模拟确定了 POR 活性位点中的多种相互作用,这些相互作用对原叶绿素内酯结合很重要,光敏化和光化学转化为叶绿素。我们证明了活性位点结构和原叶绿素内酯结构在使用 POR 变体和原叶绿素类似物的实验中驱动 POR 光化学的重要性。这些研究揭示了 POR 活性位点如何通过 NADPH 的局部氢化物转移和沿着结构定义的质子转移途径的长程质子转移促进原叶绿素的光驱动还原。蓝藻原叶绿素内酯氧化还原酶的晶体结构揭示了这种光催化活性的基础酶,通过其活性位点在光驱动下还原原叶绿素的作用。我们证明了活性位点结构和原叶绿素内酯结构在使用 POR 变体和原叶绿素类似物的实验中驱动 POR 光化学的重要性。这些研究揭示了 POR 活性位点如何通过 NADPH 的局部氢化物转移和沿结构定义的质子转移途径的长程质子转移促进原叶绿素酸的光驱动还原。蓝藻原叶绿素内酯氧化还原酶的晶体结构揭示了这种光催化活性的基础酶,通过其活性位点在光驱动下还原原叶绿素的作用。我们证明了活性位点结构和原叶绿素内酯结构在使用 POR 变体和原叶绿素类似物的实验中驱动 POR 光化学的重要性。这些研究揭示了 POR 活性位点如何通过 NADPH 的局部氢化物转移和沿结构定义的质子转移途径的长程质子转移促进原叶绿素酸的光驱动还原。蓝藻原叶绿素内酯氧化还原酶的晶体结构揭示了这种光催化活性的基础酶,通过其活性位点在光驱动下还原原叶绿素的作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号