当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A toolbox of molecular photoswitches to modulate the CXCR3 chemokine receptor with light
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-10-23 , DOI: 10.3762/bjoc.15.244 Xavier Gómez-Santacana , Sabrina M de Munnik , Tamara A M Mocking , Niels J Hauwert , Shanliang Sun , Prashanna Vijayachandran , Iwan J P de Esch , Henry F Vischer , Maikel Wijtmans , Rob Leurs
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-10-23 , DOI: 10.3762/bjoc.15.244 Xavier Gómez-Santacana , Sabrina M de Munnik , Tamara A M Mocking , Niels J Hauwert , Shanliang Sun , Prashanna Vijayachandran , Iwan J P de Esch , Henry F Vischer , Maikel Wijtmans , Rob Leurs
|
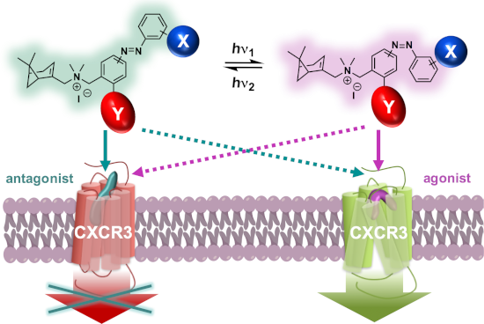
We report a detailed structure–activity relationship for the scaffold of VUF16216, a compound we have previously communicated as a small-molecule efficacy photoswitch for the peptidergic chemokine GPCR CXCR3. A series of photoswitchable azobenzene ligands was prepared through various synthetic strategies and multistep syntheses. Photochemical and pharmacological properties were used to guide the design iterations. Investigations of positional and substituent effects reveal that halogen substituents on the ortho-position of the outer ring are preferred for conferring partial agonism on the cis form of the ligands. This effect could be expanded by an electron-donating group on the para-position of the central ring. A variety of efficacy differences between the trans and cis forms emerges from these compounds. Tool compounds VUF15888 (4d) and VUF16620 (6e) represent more subtle efficacy switches, while VUF16216 (6f) displays the largest efficacy switch, from antagonism to full agonism. The compound class disclosed here can aid in new photopharmacology studies of CXCR3 signaling.
中文翻译:

分子光开关工具箱,可利用光调节CXCR3趋化因子受体
我们报告了VUF16216支架的详细的结构-活性关系,该化合物我们之前已作为肽能趋化因子GPCR CXCR3的小分子功效光开关进行了交流。通过各种合成策略和多步合成,制备了一系列可光转换的偶氮苯配体。光化学和药理特性被用来指导设计迭代。位置和取代基作用的研究表明,外环邻位的卤素取代基优选用于赋予配体顺式部分激动作用。该作用可以通过中心环对位上的给电子基团来扩展。各种药效之间的差异反式和顺式形式从这些化合物出现。工具化合物VUF15888(4d)和VUF16620(6e)表现出更微妙的功效转换,而VUF16216(6f)显示出最大的功效转换,从拮抗作用到完全激动作用。本文公开的化合物类别可有助于CXCR3信号传导的新光药理学研究。
更新日期:2019-10-24
中文翻译:

分子光开关工具箱,可利用光调节CXCR3趋化因子受体
我们报告了VUF16216支架的详细的结构-活性关系,该化合物我们之前已作为肽能趋化因子GPCR CXCR3的小分子功效光开关进行了交流。通过各种合成策略和多步合成,制备了一系列可光转换的偶氮苯配体。光化学和药理特性被用来指导设计迭代。位置和取代基作用的研究表明,外环邻位的卤素取代基优选用于赋予配体顺式部分激动作用。该作用可以通过中心环对位上的给电子基团来扩展。各种药效之间的差异反式和顺式形式从这些化合物出现。工具化合物VUF15888(4d)和VUF16620(6e)表现出更微妙的功效转换,而VUF16216(6f)显示出最大的功效转换,从拮抗作用到完全激动作用。本文公开的化合物类别可有助于CXCR3信号传导的新光药理学研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号