Cell Reports ( IF 7.5 ) Pub Date : 2019-10-22 , DOI: 10.1016/j.celrep.2019.09.040
Torben Gehring 1 , Tabea Erdmann 2 , Marco Rahm 3 , Carina Graß 1 , Andrew Flatley 4 , Thomas J O'Neill 1 , Simone Woods 1 , Isabel Meininger 1 , Ozge Karayel 5 , Kerstin Kutzner 1 , Michael Grau 2 , Hisaaki Shinohara 6 , Katja Lammens 7 , Regina Feederle 4 , Stefanie M Hauck 3 , Georg Lenz 2 , Daniel Krappmann 1
|
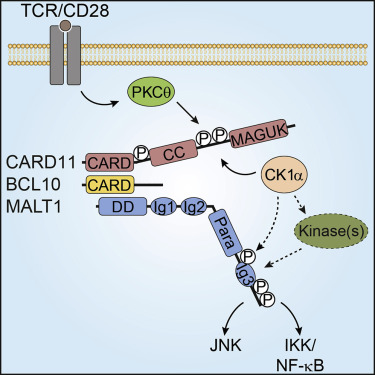
The CARMA1/CARD11-BCL10-MALT1 (CBM) complex bridges T and B cell antigen receptor (TCR/BCR) ligation to MALT1 protease activation and canonical nuclear factor κB (NF-κB) signaling. Using unbiased mass spectrometry, we discover multiple serine phosphorylation sites in the MALT1 C terminus after T cell activation. Phospho-specific antibodies reveal that CBM-associated MALT1 is transiently hyper-phosphorylated upon TCR/CD28 co-stimulation. We identify a dual role for CK1α as a kinase that is essential for CBM signalosome assembly as well as MALT1 phosphorylation. Although MALT1 phosphorylation is largely dispensable for protease activity, it fosters canonical NF-κB signaling in Jurkat and murine CD4 T cells. Moreover, constitutive MALT1 phosphorylation promotes survival of activated B cell-type diffuse large B cell lymphoma (ABC-DLBCL) cells addicted to chronic BCR signaling. Thus, MALT1 phosphorylation triggers optimal NF-κB activation in lymphocytes and survival of lymphoma cells.
中文翻译:

MALT1磷酸化控制T淋巴细胞的活化和ABC-DLBCL肿瘤细胞的存活。
CARMA1 / CARD11-BCL10-MALT1(CBM)复合物将T细胞和B细胞抗原受体(TCR / BCR)连接桥接到MALT1蛋白酶激活和典型核因子κB(NF-κB)信号传导。使用无偏质谱技术,我们发现T细胞活化后MALT1 C末端有多个丝氨酸磷酸化位点。磷酸化特异性抗体显示,与CBM相关的MALT1在TCR / CD28共同刺激后瞬时过度磷酸化。我们确定CK1α作为激酶的双重作用,这对CBM信号体装配以及MALT1磷酸化至关重要。尽管MALT1磷酸化对于蛋白酶活性而言很大程度上是可有可无的,但它可以在Jurkat和鼠CD4 T细胞中促进经典的NF-κB信号传导。而且,组成型MALT1磷酸化促进成瘾的B细胞型弥漫性大B细胞淋巴瘤(ABC-DLBCL)细胞对慢性BCR信号的依赖而存活。因此,MALT1磷酸化可触发淋巴细胞中最佳的NF-κB活化和淋巴瘤细胞的存活。

































 京公网安备 11010802027423号
京公网安备 11010802027423号